Ultra Pep-Back




Ultra Pep-Back Brand names, Ultra Pep-Back Analogs
- Alert-Pep
- Anoquan
- CFF
- Cafamil
- Cafcit
- Cafecon
- Cafeina
- Cafergot
- Caffedrine
- Caffedrine Caplets
- Caffein
- Caffeine Pure
- Caffeine, Anhydrous
- Caffeine, Monohydrate
- Caffeine, Synthetic
- Caffine
- Cafipel
- Coffein
- Coffeine
- Compound 65
- Darvon Compound
- Darvon Compound-65
- Dasin
- Dexitac
- Dexitac Stay Alert Stimulant
- Dhc Plus
- Diurex
- Durvitan
- Eldiatric C
- Enerjets
- Ercatab
- Esgic
- Esgic-Plus
- Femcet
- Fioricet
- Fiorinal
- Guaranine
- Hycomine
- Invagesic
- Invagesic Forte
- Keep Alert
- Kofein
- Koffein
- Lanorinal
- Mateina
- Maximum Strength Snapback Stimulant Powders
- Medigesic Plus
- Methyltheobromide
- Methyltheobromine
- Migergot
- Miudol
- Monomethyl Derivative of Theophylline
- Natural Caffeinum
- Nix Nap
- No-Doz
- Nodaca
- Nodoz Maximum Strength Caplets
- Norgesic
- Norgesic Forte
- Organex
- Orphengesic
- Orphengesic Forte
- Pep-Back
- Phensal
- Propoxyphene Compound 65
- Propoxyphene Compound-65
- Quick Pep
- Refresh'n
- SK 65 Compound
- Stim
- Synalgos-Dc
- Thein
- Theine
- Theobromine ME
- Theophylline ME
- Triad
- Ultra Pep-Back
- Vivarin
- Wake-Up
- Wigraine
Ultra Pep-Back Brand Names Mixture
- (Extra Strength) Acetaminophen, Caffeine & 8mg Codeine Phosphate Caplets (Acetaminophen + Caffeine + Codeine Phosphate)
- 217 (Acetylsalicylic Acid + Caffeine Citrate)
- 217 Strong Tab (Acetylsalicylic Acid + Caffeine Citrate)
- 222 Tablet (Acetylsalicylic Acid + Caffeine Citrate + Codeine Phosphate)
- 282 Mep (Acetylsalicylic Acid + Caffeine Citrate + Codeine Phosphate + Meprobamate)
- 282 Mep Tab (Acetylsalicylic Acid + Caffeine Citrate + Codeine Phosphate + Meprobamate)
- 282 Tab (Acetylsalicylic Acid + Caffeine Citrate + Codeine Phosphate)
- 282 Tablets (Acetylsalicylic Acid + Caffeine Citrate + Codeine Phosphate)
- 292 Tab (Acetylsalicylic Acid + Caffeine Citrate + Codeine Phosphate)
- 292 Tablets (Acetylsalicylic Acid + Caffeine Citrate + Codeine Phosphate)
- 692 Tab (Acetylsalicylic Acid + Caffeine + Dextropropoxyphene Hydrochloride)
- 692 Tablet (Acetylsalicylic Acid + Caffeine + Dextropropoxyphene Hydrochloride)
- a & C Tablets with Codeine 15mg (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- a & C Tablets with Codeine 30mg (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- a C and C Tab 0.125gr (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- A.C. & C 8mg Tab (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- A.C.& C Tablets (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- Ac and C Tab 1/8gr (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- Ac&C Tablets (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- Acet 2 (Acetaminophen + Caffeine + Codeine Phosphate)
- Acet 3 (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen 300 Mg with Caffeine & Codeine Caplet (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen 500mg Cmpd Tab W Codeine (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen Caffeine & 8mg Cod Phos Tab (Acetaminophen + Caffeine + Codeine (Codeine Phosphate))
- Acetaminophen Caffeine & 8mg Cod. Phos. Tab (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen Compound Caplets with Codeine (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen Compound Tablets with Codeine (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen Compound Tablets with Codeine 8mg (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen Extra Strength W Caf & Codeine (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen W Caffeine and Codeine (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen W Codeine Tab (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen with Codeine - Caplet (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen, Caffeine & 8mg Codeine Phosphate (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen, Caffeine & 8mg Codeine Phosphate Tablets (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen, Caffeine and 8 Mg of Codeine Phosphate Tablets (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophen, Caffeine, and 8mg of Codeine Phosphate Tablets (Acetaminophen + Caffeine + Codeine Phosphate)
- Acetaminophene Codeine Et Cafeine Caplets (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Acetaminophene Codeine Et Cafeine Fort (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Acetylsalicylic Acid Caffeine & 8mg Cod Phos (Acetylsalicylic Acid + Caffeine + Codeine (Codeine Phosphate))
- Acetylsalicylic Acid, Caffeine & 8 Mg Codeine Phosphate Tablets (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- Acetylsalicylic Acid, Caffeine and 8mg Codeine Phosphate (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- Alka-Seltzer Morning Relief (Acetylsalicylic Acid + Caffeine)
- Anacin Caplet (Acetylsalicylic Acid + Caffeine)
- Anacin Caplets (Acetylsalicylic Acid + Caffeine)
- Anacin Extra Strength Caplet (Acetylsalicylic Acid + Caffeine)
- Anacin Extra Strength Tablet (Acetylsalicylic Acid + Caffeine)
- Anacin Tab (Acetylsalicylic Acid + Caffeine)
- Anacin Tablet (Acetylsalicylic Acid + Caffeine)
- Anacin with Codeine Tab (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- Antidol Tablets (Acetylsalicylic Acid + Caffeine)
- Arco Pain Tab (Acetylsalicylic Acid + Caffeine Citrate)
- Asa, Caffeine & 8mg Codeine Phosphate (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- Astone Cap (Acetylsalicylic Acid + Caffeine)
- Atasol 15 Tab (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Atasol 30 Tab (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Atasol 8 Tab (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- C T Acetylsalicylic Acid Codeine and Caf Tab (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- C-2 Buffered with Codeine (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- C2 Buffered Tab (Acetylsalicylic Acid + Aluminum Hydroxide + Caffeine + Magnesium Hydroxide)
- C2 Buffered with Codeine Tab (Acetylsalicylic Acid + Aluminum Hydroxide + Caffeine + Codeine Phosphate + Magnesium Hydroxide)
- C2 Tab with Codeine (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- Cafergot Pb Sup (Belladonna + Caffeine + Ergotamine (Ergotamine Tartrate) + Pentobarbital)
- Cafergot Pb Tab (Belladonna + Caffeine + Ergotamine (Ergotamine Tartrate) + Pentobarbital Sodium)
- Cafergot Sup (Caffeine + Ergotamine Tartrate)
- Cafergot Tab (Caffeine + Ergotamine Tartrate)
- Calmine Tab (Acetylsalicylic Acid + Caffeine)
- Codamin No2 (Acetaminophen + Caffeine + Codeine Phosphate)
- Codamin No3 (Acetaminophen + Caffeine + Codeine Phosphate)
- Codaminophen Tab 1/8gr (Acetaminophen + Caffeine + Codeine Phosphate)
- Corytab (Acetaminophen + Caffeine + Diphenylpyraline Hydrochloride)
- Cotabs (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Darvon N Compound Pulvule 405 (Acetylsalicylic Acid + Caffeine + Dextropropoxyphene Napsylate)
- Dolomine 37 (Acetylsalicylic Acid + Caffeine)
- Dristan Capsules (Acetylsalicylic Acid + Caffeine + Chlorpheniramine Maleate + Phenylpropanolamine Hydrochloride)
- Emercidin D Tab (Acetaminophen + Caffeine + Diphenylpyraline Hydrochloride + Phenylpropanolamine Hydrochloride)
- Emertabs Tab (Acetaminophen + Caffeine + Phenylpropanolamine Hydrochloride)
- Ergodryl Cap (Caffeine Citrate + Diphenhydramine Hydrochloride + Ergotamine Tartrate)
- Excedrin (Acetaminophen + Caffeine)
- Excedrin Extra-Strength (Acetaminophen + Caffeine)
- Excedrin Tab (Acetaminophen + Caffeine)
- Exdol 15tab (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Exdol 30tab (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Exdol 8 Tab (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Exdol-15 Tablets (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Exdol-30 Tablets (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Exdol-8 Tablets (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Extra Strength Midol (Acetylsalicylic Acid + Caffeine)
- Fiorinal C1/2 Cap (Acetylsalicylic Acid + Butalbital + Caffeine + Codeine Phosphate)
- Fiorinal C1/4 Cap (Acetylsalicylic Acid + Butalbital + Caffeine + Codeine Phosphate)
- Fiorinal Cap (Acetylsalicylic Acid + Butalbital + Caffeine)
- Fiorinal Tab (Acetylsalicylic Acid + Butalbital + Caffeine)
- Gravergol Capsules (Caffeine + Dimenhydrinate + Ergotamine Tartrate)
- Herbopyrine Tab 325mg (Acetylsalicylic Acid + Caffeine Citrate)
- Instantine - Tab (Acetylsalicylic Acid + Caffeine)
- Instantine - Tablets (Acetylsalicylic Acid + Caffeine)
- Instantine Tab (Acetylsalicylic Acid + Caffeine)
- Madelon (Acetylsalicylic Acid + Caffeine)
- Megral Tabs (Caffeine + Cyclizine Hydrochloride + Ergotamine Tartrate)
- Midol - Caplet (Acetylsalicylic Acid + Caffeine + Cinnamedrine)
- Midol Extra Strength Gelcaps (Acetaminophen + Caffeine + Pyrilamine Maleate)
- Midol Extra Strength Menstrual (Acetaminophen + Caffeine + Pyrilamine Maleate)
- Midol Menstrual Formula - Caplet (Acetylsalicylic Acid + Caffeine)
- Midol Multi-Symptom Caplets Extra Strength (Acetaminophen + Caffeine + Pyrilamine Maleate)
- Midol Multi-Symptom Gelcap Extra Strength (Acetaminophen + Caffeine + Pyrilamine Maleate)
- Midol Regular (Acetylsalicylic Acid + Caffeine)
- Midol Tab (Acetylsalicylic Acid + Caffeine + Cinnamedrine)
- Neo Tigol Tab (Acetylsalicylic Acid + Aluminum Hydroxide + Caffeine + Magnesium Hydroxide + Passion Flower + Valerian)
- Nervine (Acetylsalicylic Acid + Caffeine)
- Nervine Tab (Acetylsalicylic Acid + Caffeine)
- Norgesic Forte Tab (Acetylsalicylic Acid + Caffeine + Orphenadrine Citrate)
- Norgesic Tab (Acetylsalicylic Acid + Caffeine + Orphenadrine Citrate)
- Novo Ac and C 8mg Tab (Acetylsalicylic Acid + Caffeine + Codeine Phosphate)
- Novo-Gesic C15 (Acetaminophen + Caffeine + Codeine Phosphate)
- Novo-Gesic C30 (Acetaminophen + Caffeine + Codeine Phosphate)
- Novo-Gesic-C8 (Acetaminophen + Caffeine + Codeine Phosphate)
- Novo-Propoxyn Compound Cap (Acetylsalicylic Acid + Caffeine + Dextropropoxyphene Hydrochloride)
- Oradrine 2 Tab (Acetaminophen + Caffeine + Diphenylpyraline Hydrochloride + Phenylpropanolamine Hydrochloride)
- Oradrine Tablets (Acetaminophen + Caffeine + Codeine Phosphate + Diphenylpyraline Hydrochloride + Phenylpropanolamine Hydrochloride)
- Oradrine-2 Tab (Acetaminophen + Caffeine + Diphenylpyraline Hydrochloride + Phenylpropanolamine Hydrochloride)
- Pain Aid (Acetylsalicylic Acid + Caffeine)
- Painex Tab 0.25gr (Acetylsalicylic Acid + Caffeine Citrate + Codeine Phosphate)
- Painex Tab 0.5gr (Acetylsalicylic Acid + Caffeine Citrate + Codeine Phosphate)
- Ratio-Lenoltec No 1 (Acetaminophen + Caffeine + Codeine Phosphate)
- Ratio-Lenoltec No 2 (Acetaminophen + Caffeine + Codeine Phosphate)
- Ratio-Lenoltec No 3 (Acetaminophen + Caffeine + Codeine Phosphate)
- Ratio-Tecnal (Acetylsalicylic Acid + Butalbital + Caffeine)
- Ratio-Tecnal C 1/2 (Acetylsalicylic Acid + Butalbital + Caffeine + Codeine Phosphate)
- Ratio-Tecnal C1/4 (Acetylsalicylic Acid + Butalbital + Caffeine + Codeine Phosphate)
- Sinugex 38 (Acetaminophen + Caffeine + Diphenylpyraline Hydrochloride)
- Triaminicin Colds & Flu Caplets (Acetaminophen + Caffeine + Pheniramine Maleate + Phenylpropanolamine Hydrochloride + Pyrilamine Maleate)
- Trianal Capsules (Acetylsalicylic Acid + Butalbital + Caffeine)
- Trianal Tablet (Acetylsalicylic Acid + Butalbital + Caffeine)
- Trianal-C 1/2 Capsule (Acetylsalicylic Acid + Butalbital + Caffeine + Codeine Phosphate)
- Triatec-8 Fort Tab (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Triatec-8 Tab (Acetaminophen + Caffeine Citrate + Codeine Phosphate)
- Tylenol No 1 Tab (Acetaminophen + Caffeine + Codeine Phosphate)
- Tylenol No.1 Caplets (Acetaminophen + Caffeine + Codeine Phosphate)
- Tylenol No.1 Forte Caplets (Acetaminophen + Caffeine + Codeine Phosphate)
- Tylenol No1 Forte Tablets with Codeine (Acetaminophen + Caffeine + Codeine Phosphate)
- Tylenol Ultra Relief (Acetaminophen + Caffeine)
- Tylenol W Codeine No2 Tab (Acetaminophen + Caffeine + Codeine Phosphate)
- Tylenol W Codeine No3 Tab (Acetaminophen + Caffeine + Codeine Phosphate)
- Tylenol with Codeine No. 2 - Tab (Acetaminophen + Caffeine + Codeine Phosphate)
- Tylenol with Codeine No. 3 - Tab (Acetaminophen + Caffeine + Codeine Phosphate)
- Ultra Headache Relief (Acetaminophen + Caffeine)
- Wigraine Suppositories (Belladonna + Caffeine + Ergotamine Tartrate)
- Wigraine Tab (Belladonna + Caffeine + Ergotamine Tartrate)
Ultra Pep-Back Chemical_Formula
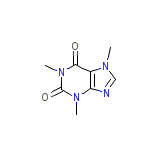
C8H10N4O2
Ultra Pep-Back RX_link
http://www.rxlist.com/cgi/generic3/caffeine.htm
Ultra Pep-Back fda sheet
Ultra Pep-Back msds (material safety sheet)
Ultra Pep-Back Synthesis Reference
U.S. Pat. 2,508,545 (1950)
Ultra Pep-Back Molecular Weight
194.191 g/mol
Ultra Pep-Back Melting Point
238 oC
Ultra Pep-Back H2O Solubility
22 mg/ml
Ultra Pep-Back State
Solid
Ultra Pep-Back LogP
-0.07
Ultra Pep-Back Dosage Forms
Capsule; Drops; Liquid; Pill; Tablet
Ultra Pep-Back Indication
For management of fatigue, orthostatic hypotension, and for the short term treatment of apnea of prematurity in infants.
Ultra Pep-Back Pharmacology
Caffeine, a naturally occurring xanthine derivative like theobromine and the bronchodilator theophylline, is used as a CNS stimulant, mild diuretic, and respiratory stimulant (in neonates with apnea of prematurity). Often combined with analgesics or with ergot alkaloids, caffeine is used to treat migraine and other headache types. Over the counter, caffeine is available to treat drowsiness or mild water-weight gain.
Ultra Pep-Back Absorption
Readily absorbed after oral or parenteral administration. The peak plasma level for caffeine range from 6-10mg/L and the mean time to reach peak concentration ranged from 30 minutes to 2 hours.
Ultra Pep-Back side effects and Toxicity
LD50=127 mg/kg (orally in mice)
Ultra Pep-Back Patient Information
Ultra Pep-Back Organisms Affected
Humans and other mammals