Rozon




Rozon Brand names, Rozon Analogs
- Alquen
- Alter-H2
- Alvidina
- Apo-Ranitidin
- Artomil
- Azuranit
- Coralen [inj.]
- Digestosan
- Ergan
- Esofex
- Fendibina
- Gastrial
- Gastridina
- Gastrolav
- Gastrosedol
- Kuracid
- Label
- Lake
- Logat
- Melfax
- Microtid
- Mideran
- Neugal
- Noctone
- Noktome
- Normon
- Novo-Radinine
- Nu-Ranit
- Pep-Rani
- Ptinolin
- Quadrin
- Quantor [inj.]
- RAN
- Radin
- Ran H2
- Ran Lich
- Rani 2
- Rani AbZ
- Rani-BASF
- Rani-Puren
- Rani-Q
- Rani-Sanorania
- Rani-nerton
- Raniben
- Raniberl
- Raniberta
- Ranibloc
- Ranic
- Ranicux
- Ranidil
- Ranidin
- Ranidine
- Ranidura
- Ranifur [inj.]
- Ranigasan
- Ranigast
- Ranigen
- Ranilonga
- Ranimerck
- Raniogas
- Raniplex
- Ranisan
- Ranitab
- Ranitic
- Ranitidin
- Ranitidin 1A Pharma
- Ranitidin AL
- Ranitidin AWD
- Ranitidin Arcana
- Ranitidin Atid
- Ranitidin Basics
- Ranitidin Duncan
- Ranitidin Dyna
- Ranitidin Helvepharm
- Ranitidin Heumann
- Ranitidin Hexal
- Ranitidin Merck
- Ranitidin Millet
- Ranitidin NM
- Ranitidin Normon
- Ranitidin PB
- Ranitidin Stada
- Ranitidin von ct
- Ranitidin-Cophar
- Ranitidin-Isis
- Ranitidin-ratiopharm
- Ranitidina Tamarang
- Ranitidina predilu Grif
- Ranitidine Base
- Ranitidine HCL
- Ranitidine HCl (Form I And Form Ii)
- Ranitidine hydrochloride
- Ranitidine hydrochloride (JP14/USP)
- Ranitidine hydrochloride [JAN]
- Ranitiget
- Ranitin
- Ranitine
- Ranobel
- Rantacid
- Rantidine HCL
- Ranuber
- Raticina
- Regalil
- Renatac
- Rozon
- Rubiulcer
- Santanol
- Serviradine
- Sostril
- Tanidina
- Taural
- Terposen
- Toriol
- Trigger
- Ulcecur
- Ulcex
- Ulcirex
- Ulcodin
- Ulcolind Rani
- Ulcosan
- Ulsaven
- Ultidine
- Viserul
- Zandid
- Zantac
- Zantac 150
- Zantac 75
- Zantac In Plastic Container
- Zantarac
- Zantic
Rozon Brand Names Mixture
- Tritec (ranitidine bismuth citrate salt)
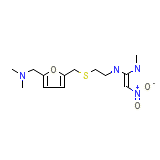
Rozon Chemical_Formula
C13H22N4O3S
Rozon RX_link
http://www.rxlist.com/cgi/generic2/ranitbc.htm
Rozon fda sheet
Rozon msds (material safety sheet)
Rozon Synthesis Reference
B. J. Price et al., U.S. Pat. 4,128,658 (1978)
Rozon Molecular Weight
314.405 g/mol
Rozon Melting Point
69-70 oC
Rozon H2O Solubility
24.7 mg/mL
Rozon State
Solid
Rozon LogP
1.93
Rozon Dosage Forms
Capsule; Liquid; Solution; Tablet; Syrup
Rozon Indication
Used in the treatment of peptic ulcer disease (PUD), dyspepsia, stress ulcer prophylaxis, and gastroesophageal reflux disease (GERD).
Rozon Pharmacology
Ranitidine is a histamine H2-receptor antagonist similar to cimetidine and famotidine. An H2-receptor antagonist, often shortened to H2 antagonist, is a drug used to block the action of histamine on parietal cells in the stomach, decreasing acid production by these cells. These drugs are used in the treatment of dyspepsia, however their use has waned since the advent of the more effective proton pump inhibitors. Like the H1-antihistamines, the H2 antagonists are inverse agonists rather than true receptor antagonists.
Rozon Absorption
Approximately 50% bioavailability orally.
Rozon side effects and Toxicity
LD50=77mg/kg (orally in mice). Symptoms of overdose include muscular tremors, vomiting, and rapid respiration.
Rozon Patient Information
PATIENT INFORMATION
Phenylketonurics: ZANTAC 25 EFFERdose Tablets contain phenylalanine 2.81 mg per 25 mg of ranitidine. ZANTAC 150 EFFERdose Tablets
contain phenylalanine 16.84 mg per 150 mg of ranitidine. ZANTAC EFFERdose Tablets should not be chewed, swallowed whole, or dissolved
on the tongue.
Phenylketonurics: ZANTAC 25 EFFERdose Tablets contain phenylalanine 2.81 mg per 25 mg of ranitidine. ZANTAC 150 EFFERdose Tablets
contain phenylalanine 16.84 mg per 150 mg of ranitidine. ZANTAC EFFERdose Tablets should not be chewed, swallowed whole, or dissolved
on the tongue.
Rozon Organisms Affected
Humans and other mammals