Restadin




Restadin Brand names, Restadin Analogs
- Amfamox
- Antodine
- Apo-Famotidine
- Apogastine
- Bestidine
- Blocacid
- Brolin
- Cepal
- Confobos
- Cronol
- Cuantin
- Dibrit 40
- Digervin
- Dinul
- Dipsin
- Dispromil
- Dispronil
- Duovel
- Durater
- Evatin
- Fadin
- Fadine
- Fadyn
- Fagastine
- Famo
- Famocid
- Famodar
- Famodil
- Famodin
- Famodine
- Famogard
- Famonit
- Famopsin
- Famos
- Famosan
- Famotal
- Famotep
- Famotidina [Spanish]
- Famotidinum [Latin]
- Famotin
- Famovane
- Famowal
- Famox
- Famoxal
- Famtac
- Famulcer
- Fanobel
- Fanosin
- Fanox
- Farmotex
- Ferotine
- Fibonel
- Fluxid
- Fudone
- Ganor
- Gaster
- Gastridan
- Gastridin
- Gastrion
- Gastro
- Gastrodomina
- Gastrofam
- Gastropen
- Gastrosidin
- H2 Bloc
- Hacip
- Huberdina
- Ingastri
- Invigan
- Lecedil
- Logos
- Mensoma
- Midefam
- Mosul
- Motiax
- Muclox
- Mylanta AR
- Neocidine
- Nevofam
- Notidin
- Novo-Famotidine
- Nu-Famotidine
- Nulceran
- Nulcerin
- Panalba
- Pepcid
- Pepcid AC
- Pepcid Ac (geltab)
- Pepcid RPD
- Pepcidin
- Pepcidin Rapitab
- Pepcidina
- Pepcidine
- Pepdif
- Pepdine
- Pepdul
- Pepfamin
- Peptan
- Peptidin
- Peptifam
- Pepzan
- Purifam
- Quamatel
- Quamtel
- Renapepsa
- Restadin
- Rogasti
- Rubacina
- Sedanium-R
- Sigafam
- Supertidine
- Tairal
- Tamin
- Tipodex
- Topcid
- Ulcatif
- Ulceprax
- Ulcofam
- Ulfagel
- Ulfam
- Ulfamid
- Ulfinol
- Ulgarine
- Vagostal
- Weimok
- Whitidin
- Yamarin
Restadin Brand Names Mixture
- No information avaliable
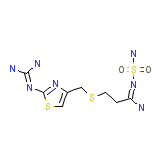
Restadin Chemical_Formula
C8H15N7O2S3
Restadin RX_link
http://www.rxlist.com/cgi/generic/famot.htm
Restadin fda sheet
Restadin msds (material safety sheet)
Restadin Synthesis Reference
H. Yasufumi et al.; U.S. Pat. 4,283,408 (1981)
Restadin Molecular Weight
337.449 g/mol
Restadin Melting Point
163-164 oC
Restadin H2O Solubility
1.1 mg/mL
Restadin State
Solid
Restadin LogP
-0.726
Restadin Dosage Forms
Oral tablets; Rapid disintergrating tablets; Suspension; Injectable solution
Restadin Indication
For the treatment of peptic ulcer disease (PUD) and gastroesophageal reflux disease (GERD).
Restadin Pharmacology
Famotidine, a competitive histamine H2-receptor antagonist, is used to treat gastrointestinal disorders such as gastric or duodenal ulcer, gastroesophageal reflux disease, and pathological hypersecretory conditions. Famotidine inhibits many of the isoenzymes of the hepatic CYP450 enzyme system. Other actions of Famotidine include an increase in gastric bacterial flora such as nitrate-reducing organisms.
Restadin Absorption
The bioavailability of oral doses is 40-45%.
Restadin side effects and Toxicity
Intravenous, mouse: LD50 = 244.4mg/kg; Oral, mouse: LD50 = 4686 mg/kg. Symptoms of overdose include emesis, restlessness, pallor of mucous membranes or redness of mouth and ears, hypotension, tachycardia and collapse.
Restadin Patient Information
Famotidine is used to treat stomach and duodenal (upper small intestine) ulcers;
hypersecretory (increased acid secretion) conditions; heartburn and gastroesophageal
reflux disease (stomach contents bubbling into the esophagus causing pain). Notify your
physician if you are pregnant or nursing. Famotidine may be taken with or without food.
Shake the oral suspension vigorously for 5-10 seconds before taking. Unused oral
suspension should be discarded after 30 days. Notify your physician if you develop black,
tarry stools or coffee-ground vomit.
hypersecretory (increased acid secretion) conditions; heartburn and gastroesophageal
reflux disease (stomach contents bubbling into the esophagus causing pain). Notify your
physician if you are pregnant or nursing. Famotidine may be taken with or without food.
Shake the oral suspension vigorously for 5-10 seconds before taking. Unused oral
suspension should be discarded after 30 days. Notify your physician if you develop black,
tarry stools or coffee-ground vomit.
Restadin Organisms Affected
Humans and other mammals