Epsikapron




Epsikapron Brand names, Epsikapron Analogs
Epsikapron Brand Names Mixture
- No information avaliable
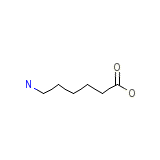
Epsikapron Chemical_Formula
C6H13NO2
Epsikapron RX_link
http://www.rxlist.com/cgi/generic/amicarpre.htm
Epsikapron fda sheet
Epsikapron msds (material safety sheet)
Epsikapron Synthesis Reference
No information avaliable
Epsikapron Molecular Weight
131.173 g/mol
Epsikapron Melting Point
205 oC
Epsikapron H2O Solubility
5.05E+005 mg/L
Epsikapron State
Solid
Epsikapron LogP
0.306
Epsikapron Dosage Forms
Solution for injection (containing 250 mg/mL of aminocaproic acid) and tablets (500, 1000mg)
Epsikapron Indication
For use in the treatment of excessive postoperative bleeding.
Epsikapron Pharmacology
Aminocaproic acid works as an antifibrinolytic. It is a derivative of the amino acid lysine. The fibrinolysis-inhibitory effects of aminocaproic acid appear to be exerted principally via inhibition of plasminogen activators and to a lesser degree through antiplasmin activity.
Epsikapron Absorption
Absorbed rapidly following oral administration. In adults, oral absorption appears to be a zero-order process with an absorption rate of 5.2 g/hr. The mean lag time in absorption is 10 minutes. After a single oral dose of 5 g, absorption was complete (F=1).
Epsikapron side effects and Toxicity
A few cases of acute overdosage with intravenous administration have been reported. The effects have ranged from no reaction to transient hypotension to severe acute renal failure leading to death. The intravenous and oral LD50 were 3.0 and 12.0 g/kg respectively in the mouse and 3.2 and 16.4 g/kg respectively in the rat. An intravenous infusion dose of 2.3 g/kg was lethal in the dog.
Epsikapron Patient Information
Epsikapron Organisms Affected
Humans and other mammals