Urbilat




Urbilat Brand names, Urbilat Analogs
- 3P bamate
- Amepromat
- Amosene
- Anastress
- Anathylmon
- Anatimon
- Andaksin
- Andaxin
- Aneural
- Aneurol
- Aneusral
- Aneuxal
- Aneuxral
- Ansiatan
- Ansietan
- Ansil
- Ansiowas
- Anural
- Anxietil
- Anzil
- Apascil
- Apasil
- Apo-Meprobamate
- Appetrol
- Appetrol-Sr
- Arcoban
- Arpon
- Artolon
- Ataraxine
- Atraxin
- Auxietil
- Ayeramate
- Ayermate
- Bamate
- Bamd 400
- Bamo 400
- Biobamat
- Biobamate
- Brobamate
- Calmadin
- Calmax
- Calmiren
- Canquil 400
- Canquil-400
- Carbaxin
- Cirpon
- Cirponyl
- Coprobate
- Crestanil
- Cypron
- Cyrpon
- DEA No. 2820
- Dapaz
- Deprol
- Despasmol
- Dicandiol
- Diron
- Diurnal
- Diurnaldiverondormabrol
- Diveron
- Dormabrol
- Ecuanil
- Edenal
- Enorden
- Epicur
- Epikur
- Equagesic
- Equanil
- Equanil suspension
- Equatrate
- Equazine-M
- Equilium
- Equinil
- Equitar
- Erina
- Estasil
- Fas-Cile
- Fas-Cile 200
- Gadexyl
- Gagexyl
- Harmonin
- Hartol
- Holbamate
- Ipsotian
- Kesso-Bamate
- Kessobamate
- Klort
- Larten
- Lepenil
- Lepetown
- Letyl
- Libiolan
- Madiol
- Mar-Bate
- Margonil
- Mendel
- Mepamtin
- Mepantin
- Mepavlon
- Mepiosine
- Meposed
- Mepranil
- Mepriam
- Meprin
- Meprindon
- Mepro-Aspirin
- Mepro-analgesic
- Meprobam
- Meprobamat
- Meprobamat [German]
- Meprobamate [USAN:BAN:INN:JAN]
- Meprobamate and Aspirin Tablets
- Meprobamato
- Meprobamato [INN-Spanish]
- Meprobamato [Italian]
- Meprobamatum [INN-Latin]
- Meproban
- Meprocompren
- Meprocon
- Meprocon CMC
- Meprodil
- Meprodiol
- Meprol
- Meproleaf
- Mepron
- Mepronil
- Meprosa
- Meprosan
- Meprosin
- Meprospan
- Meprospan 200
- Meprospan 400
- Meprospan-200
- Meprospan-400
- Meprotabs
- Meprotan
- Meprotanum
- Meproten
- Meprotil
- Meprovan
- Meprovanmeprozine
- Meprozine
- Meptran
- Meptranactylmilprem
- Mesmar
- Metractyl
- Metranquil
- Micrainin
- Milpath
- Milprem
- Milprem-200
- Milprem-400
- Miltamato
- Miltann
- Miltaun
- Miltown
- Miltown 600
- Miltown-200
- Miltown-400
- Miltown-600
- Miltrate
- Miltuan
- Miltwon
- Misedant
- Morbam
- Multaun
- My-trans
- Neo-Tran
- Nephentine
- Nervonus
- Neuramate
- Oasil
- Optarket
- Orlevol
- Orolevol
- Pan-tranquil
- Pancalma
- Panediol
- Pankalma
- Pathibamate
- Paxin
- Pensive
- Perequietil
- Perequil
- Perquietil
- Pertranquil
- Pimal
- Placidon
- Placitate
- Prequil
- Probamato
- Probamyl
- Probate
- Procalmadiol
- Procalmadol
- Procalmidol
- Procarbamide
- Promate
- Promato
- Proquanil
- Protran
- Q-Gesic
- Quaname
- Quanane
- Quanil
- Quietidon
- Quivet
- Rastenil
- Reostral
- Restenil
- Restenyl
- Restinal
- Restinil
- Restran
- Robamate
- SK-Bamate
- Sadanyl
- Scolazil
- Sedabamate
- Sedanil
- Sedanyl
- Sedazil
- Sedoquil
- Sedoselecta
- Selene
- Seril
- Setran
- Shalvaton
- Solevione anastress
- Sowell
- Spantran
- Stensolo
- Tamate
- Tensol
- Tensonal
- Trancot
- Trankvilan
- Tranlisant
- Tranmep
- Tranquil
- Tranquilan
- Tranquilate
- Tranquilax
- Tranquiline
- Tranquillin
- Tranquilsan
- Tranquinol
- Tranquisan
- Trelmar
- Urbil
- Urbilat
- Vio-Bamate
- Vistabamate
- Wardamate
- Wyseals
- Zirpon
Urbilat Brand Names Mixture
- No information avaliable
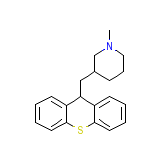
Urbilat Chemical_Formula
C20H23NS
Urbilat RX_link
No information avaliable
Urbilat fda sheet
Urbilat msds (material safety sheet)
Urbilat Synthesis Reference
No information avaliable
Urbilat Molecular Weight
309.469 g/mol
Urbilat Melting Point
< 25 oC
Urbilat H2O Solubility
Soluble as HCl salt
Urbilat State
Liquid
Urbilat LogP
5.7
Urbilat Dosage Forms
Tablets (oral, 1 mg)
Urbilat Indication
Used for the symptomatic treatment of parkinsonism.
Urbilat Pharmacology
Metixene is a tertiary antimuscarinic with actions similar to those of atropine; it also has antihistaminic and direct antispasmodic properties. It is used for the symptomatic treatment of parkinsonism, including the alleviation of the extrapyramidal syndrome induced by other drugs such as phenothiazines, but, like other antimuscarinics, it is of no value against tardive dyskinesias. Metixene has been discontinued.
Urbilat Absorption
Absorbed in the gastrointestinal tract following oral administration, however the extent of absorption is not known.
Urbilat side effects and Toxicity
Signs of overdose include dilated and sluggish pupils, warm, dry skin, facial flushing, decreased secretions of the mouth, pharynx, nose, and bronchi, foul-smelling breath, elevated temperature, tachycardia, cardiac arrhythmias, decreased bowel sounds, urinary retention, delirium, disorientation, anxiety, hallucinations, illusions, confusion, incoherence, agitation, hyperactivity, ataxia, loss of memory, paranoia, combativeness, and seizures.
Urbilat Patient Information
Urbilat Organisms Affected
Humans and other mammals