Mepron




Mepron Brand names, Mepron Analogs
Mepron Brand Names Mixture
- No information avaliable
Mepron Chemical_Formula
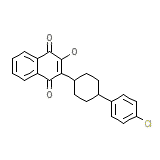
C22H19ClO3
Mepron RX_link
http://www.rxlist.com/cgi/generic/atovaqu.htm
Mepron fda sheet
Mepron msds (material safety sheet)
Mepron Synthesis Reference
No information avaliable
Mepron Molecular Weight
366.837 g/mol
Mepron Melting Point
No information avaliable
Mepron H2O Solubility
Practically insoluble
Mepron State
Solid
Mepron LogP
5.251
Mepron Dosage Forms
Suspension; Tablet
Mepron Indication
For the treatment or prevention of Pneumocystis carinii pneumonia in patients who are intolerant to trimethoprim-sulfamethoxazole (TMP-SMX). Also indicated for the acute oral treatment of mild to moderate PCP in patients who are intolerant to TMP-SMX.
Mepron Pharmacology
Atovaquone is a highly lipophilic drug that closely resembles the structure ubiquinone. Its inhibitory effect being comparable to ubiquinone, in sensitive parasites atovaquone can act by selectively affecting mitochondrial electron transport and parallel processes such as ATP and pyrimidine biosynthesis. For illustration, cytochrome bc1 complex (complex III) seems to serve as a highly discriminating molecular target for atovaquone in Plasmodia
Mepron Absorption
The bioavailability of atovaquone is low and variable and is highly dependent on formulation and diet. Bioavailability of the suspension increases two-fold when administered with meals. When administered with food, bioavailability is approximately 47%. Without food, the bioavailability is 23%.
Mepron side effects and Toxicity
The median lethal dose is higher than the maximum oral dose tested in mice and rats (1825 mg/kg per day). Overdoses up to 31,500 mg of atovaquone have been reported. In one such patient who also took an unspecified dose of dapsone, methemoglobinemia occurred. Rash has also been reported after overdose.
Mepron Patient Information
Mepron Organisms Affected
Plasmodium and other