Vincamin compositum




Vincamin compositum Brand names, Vincamin compositum Analogs
- Abitrate
- Amotril
- Amotril S
- Angiokapsul
- Anparton
- Antilipid
- Antilipide
- Apolan
- Arterioflexin
- Arterosol
- Artes
- Artevil
- Ateculon
- Ateriosan
- Athebrate
- Atheromide
- Atheropront
- Athranid-Wirkstoff
- Atrolen
- Atromid
- Atromid S
- Atromid-S
- Atromida
- Atromidin
- Atrovis
- Azionyl
- Bioscleran
- Bresit
- CPIB
- Cartagyl
- Chlorfenisate
- Chlorophenoxyisobutyricacidethylester
- Chlorphenisate
- Cinnarizin
- Cinnarizine
- Citiflus
- Claripex
- Claripex CPIB
- Cloberat
- Clobrat
- Clobren-5F
- Clobren-SF
- Clofar
- Clofibate
- Clofibram
- Clofibrat
- Clofibrato
- Clofibratum
- Clofinit
- Clofipront
- Delipid
- Deliva
- Dura clofibrat
- ELPI
- EPIB
- Ethyl chlorophenoxyisobutyrate
- Ethyl clofibrate
- Ethyl p-chlorophenoxyisobutyrate
- Ethyl para-chlorophenoxyisobutyrate
- Fibralem
- Gerastop
- Hyclorate
- Klofibrat
- Klofiran
- Levatrom
- Lipamid
- Lipavil
- Lipavlon
- Lipide 500
- Lipidsenker
- Lipofacton
- Lipomid
- Liponorm
- Liporeduct
- Liporil
- Liposid
- Liprin
- Liprinal
- Lobetrin
- Miscleron
- Negalip
- Neo-Atromid
- Normalip
- Normat
- Normet
- Normolipol
- Novofibrate
- Oxan 600
- Persantinat
- Recolip
- Regardin
- Regelan
- Regelan N
- Robigram
- Scrobin
- Serofinex
- Serotinex
- Skerolip
- Sklerepmexe
- Sklero
- Sklero-Tablinen
- Sklero-tablinene
- Sklero-tabuls
- Sklerolip
- Skleromex
- Skleromexe
- Tetrasipton
- Thillate
- Thiosan
- Ticlobran
- Vincamin compositum
- Xyduril
- Yoclo
- neo-Atomid
Vincamin compositum Brand Names Mixture
- No information avaliable
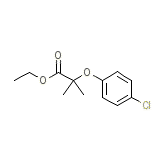
Vincamin compositum Chemical_Formula
C12H15ClO3
Vincamin compositum RX_link
http://www.rxlist.com/cgi/generic2/clofibrate.htm
Vincamin compositum fda sheet
Vincamin compositum msds (material safety sheet)
Vincamin compositum Synthesis Reference
No information avaliable
Vincamin compositum Molecular Weight
242.698 g/mol
Vincamin compositum Melting Point
< 25 oC (boiling point 148-150°C at 25 mm Hg)
Vincamin compositum H2O Solubility
Insoluble
Vincamin compositum State
Liquid
Vincamin compositum LogP
3.58
Vincamin compositum Dosage Forms
Capsules (orange, oblong, soft-gelatin capsules, each containing 500 mg clofibrate)
Vincamin compositum Indication
For Primary Dysbetalipoproteinemia (Type III hyperlipidemia) that does not respond adequately to diet.
Vincamin compositum Pharmacology
Clofibrate is an antilipidemic agent similar to gemfibrozil. It acts to lower elevated serum lipids by reducing the very low-density lipoprotein fraction (Sf 20-400) rich in triglycerides. Serum cholesterol may be decreased, particularly in those patients whose cholesterol elevation is due to the presence of IDL as a result of Type III hyperlipoproteinemia. Several investigators have observed in their studies that clofibrate may produce a decrease in cholesterol linoleate but an increase in palmitoleate and oleate, the latter being considered atherogenic in experimental animals. The significance of this finding is unknown at this time. Reduction of triglycerides in some patients treated with clofibrate or certain of its chemically and clinically similar analogs may be associated with an increase in LDL cholesterol. Increase in LDL cholesterol has been observed in patients whose cholesterol is initially normal. Animal studies suggest that clofibrate interrupts cholesterol biosynthesis prior to mevalonate formation.
Vincamin compositum Absorption
Completely but slowly absorbed from the intestine. Between 95% and 99% of an oral dose of clofibrate is excreted in the urine as free and conjugated clofibric acid; thus, the absorption of clofibrate is virtually complete.
Vincamin compositum side effects and Toxicity
Oral, mouse: LD50 = 1220 mg/kg; Oral, rabbit: LD50 = 1370 mg/kg; Oral, rat: LD50 = 940 mg/kg. No reported case of overdosage in humans.
Vincamin compositum Patient Information
Vincamin compositum Organisms Affected
Humans and other mammals