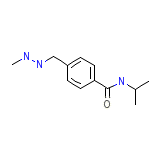
Ibenzmethyzine hydrochloride




Ibenzmethyzine hydrochloride Brand names, Ibenzmethyzine hydrochloride Analogs
Ibenzmethyzine hydrochloride Brand Names Mixture
- No information avaliable
Ibenzmethyzine hydrochloride Chemical_Formula
C12H19N3O
Ibenzmethyzine hydrochloride RX_link
http://www.rxlist.com/cgi/generic3/procarb.htm
Ibenzmethyzine hydrochloride fda sheet
Ibenzmethyzine hydrochloride msds (material safety sheet)
Ibenzmethyzine hydrochloride Synthesis Reference
No information avaliable
Ibenzmethyzine hydrochloride Molecular Weight
221.299 g/mol
Ibenzmethyzine hydrochloride Melting Point
223 oC
Ibenzmethyzine hydrochloride H2O Solubility
1420 mg/L
Ibenzmethyzine hydrochloride State
Solid
Ibenzmethyzine hydrochloride LogP
1.234
Ibenzmethyzine hydrochloride Dosage Forms
Capsule
Ibenzmethyzine hydrochloride Indication
For use with other anticancer drugs for the treatment of stage III and stage IV Hodgkin's disease.
Ibenzmethyzine hydrochloride Pharmacology
Procarbazine is an antineoplastic in the class of alkylating agents and is used to treat various forms of cancer. Alkylating agents are so named because of their ability to add alkyl groups to many electronegative groups under conditions present in cells. They stop tumor growth by cross-linking guanine bases in DNA double-helix strands - directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. In addition, these drugs add methyl or other alkyl groups onto molecules where they do not belong which in turn inhibits their correct utilization by base pairing and causes a miscoding of DNA. Procarbazine is cell-phase specific for the S phase of cell division.
Ibenzmethyzine hydrochloride Absorption
Procarbazine is rapidly and completely absorbed.
Ibenzmethyzine hydrochloride side effects and Toxicity
LD50=785 mg/kg (orally in rats)
Ibenzmethyzine hydrochloride Patient Information
Ibenzmethyzine hydrochloride Organisms Affected
Humans and other mammals