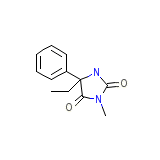
Sedantional




Sedantional Brand names, Sedantional Analogs
Sedantional Brand Names Mixture
- No information avaliable
Sedantional Chemical_Formula
C12H14N2O2
Sedantional RX_link
No information avaliable
Sedantional fda sheet
Sedantional msds (material safety sheet)
Sedantional Synthesis Reference
No information avaliable
Sedantional Molecular Weight
218.252 g/mol
Sedantional Melting Point
135 oC
Sedantional H2O Solubility
1270 mg/L
Sedantional State
Solid
Sedantional LogP
1.515
Sedantional Dosage Forms
Tablet
Sedantional Indication
For the treatment of refractory partial epilepsy.
Sedantional Pharmacology
Mephenytoin is an antiepileptic drug which can be useful in the treatment of epilepsy. The primary site of action appears to be the motor cortex where spread of seizure activity is inhibited. Possibly by promoting sodium efflux from neurons, mephenytoin tends to stabilize the threshold against hyperexcitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient. This includes the reduction of posttetanic potentiation at synapses. Loss of posttetanic potentiation prevents cortical seizure foci from detonating adjacent cortical areas. Mephenytoin reduces the maximal activity of brain stem centers responsible for the tonic phase of tonic-clonic (grand mal) seizures.
Sedantional Absorption
No information avaliable
Sedantional side effects and Toxicity
No information avaliable
Sedantional Patient Information
Patient Information:
Discuss with your doctor about the risks of taking Mephenytoin. Mephenytoin may
pass into the breast milk. If you are breast feeding or have any other medical condition,
make sure you let the doctor know.
Alcohol and some other medications may affect the effect of Mephenytoin.
Do not change dose and dosing schedule without consulting your doctor.
Discuss with your doctor about the risks of taking Mephenytoin. Mephenytoin may
pass into the breast milk. If you are breast feeding or have any other medical condition,
make sure you let the doctor know.
Alcohol and some other medications may affect the effect of Mephenytoin.
Do not change dose and dosing schedule without consulting your doctor.
Sedantional Organisms Affected
Humans and other mammals