Sacerno




Sacerno Brand names, Sacerno Analogs
Sacerno Brand Names Mixture
- No information avaliable
Sacerno Chemical_Formula
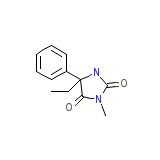
C12H14N2O2
Sacerno RX_link
No information avaliable
Sacerno fda sheet
Sacerno msds (material safety sheet)
Sacerno Synthesis Reference
No information avaliable
Sacerno Molecular Weight
218.252 g/mol
Sacerno Melting Point
135 oC
Sacerno H2O Solubility
1270 mg/L
Sacerno State
Solid
Sacerno LogP
1.515
Sacerno Dosage Forms
Tablet
Sacerno Indication
For the treatment of refractory partial epilepsy.
Sacerno Pharmacology
Mephenytoin is an antiepileptic drug which can be useful in the treatment of epilepsy. The primary site of action appears to be the motor cortex where spread of seizure activity is inhibited. Possibly by promoting sodium efflux from neurons, mephenytoin tends to stabilize the threshold against hyperexcitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient. This includes the reduction of posttetanic potentiation at synapses. Loss of posttetanic potentiation prevents cortical seizure foci from detonating adjacent cortical areas. Mephenytoin reduces the maximal activity of brain stem centers responsible for the tonic phase of tonic-clonic (grand mal) seizures.
Sacerno Absorption
No information avaliable
Sacerno side effects and Toxicity
No information avaliable
Sacerno Patient Information
Patient Information:
Discuss with your doctor about the risks of taking Mephenytoin. Mephenytoin may
pass into the breast milk. If you are breast feeding or have any other medical condition,
make sure you let the doctor know.
Alcohol and some other medications may affect the effect of Mephenytoin.
Do not change dose and dosing schedule without consulting your doctor.
Discuss with your doctor about the risks of taking Mephenytoin. Mephenytoin may
pass into the breast milk. If you are breast feeding or have any other medical condition,
make sure you let the doctor know.
Alcohol and some other medications may affect the effect of Mephenytoin.
Do not change dose and dosing schedule without consulting your doctor.
Sacerno Organisms Affected
Humans and other mammals