Eurosan




Eurosan Brand names, Eurosan Analogs
- Alboral
- Aliseum
- Alupram
- Amiprol
- An-Ding
- Ansiolin
- Ansiolisina
- Apaurin
- Apo-Diazepam
- Apozepam
- Armonil
- Assival
- Atensine
- Atilen
- Bensedin
- Bialzepam
- Calmocitene
- Calmpose
- Cercine
- Ceregulart
- Condition
- DAP
- Diacepan
- Dialag
- Dialar
- Diapam
- Diastat
- Diazemuls
- Diazemulus
- Diazepam Intensol
- Diazepan
- Diazetard
- Dienpax
- Dipam
- Dipezona
- Dizac
- Domalium
- Duksen
- Duxen
- E-Pam
- Eridan
- Eurosan
- Evacalm
- Faustan
- Faustan,
- Freudal
- Frustan
- Gewacalm
- Gihitan
- Horizon
- Kabivitrum
- Kiatrium
- LA III
- La-Iii
- Lamra
- Lembrol
- Levium
- Liberetas
- Mandrozep
- Methyldiazepinone
- Methyldiazepinone, Pharmaceutical
- Morosan
- Neurolytril
- Noan
- Novazam
- Novo-Dipam
- Paceum
- Pacitran
- Paranten
- Paxate
- Paxel
- Plidan
- Pms-Diazepam
- Pro-Pam
- Q-Pam
- Q-Pam Relanium
- Quetinil
- Quiatril
- Quievita
- Relaminal
- Relanium
- Relax
- Renborin
- Ruhsitus
- S.A. R.L.
- Saromet
- Sedapam
- Sedipam
- Seduksen
- Seduxen
- Serenack
- Serenamin
- Serenzin
- Servizepam
- Setonil
- Sibazon
- Sibazone
- Solis
- Sonacon
- Stesolid
- Stesolin
- Tensopam
- Tranimul
- Tranqdyn
- Tranquase
- Tranquirit
- Tranquo-Puren
- Tranquo-Tablinen
- Umbrium
- Unisedil
- Usempax Ap
- Valaxona
- Valeo
- Valiquid
- Valitran
- Valium
- Valrelease
- Vatran
- Velium
- Vival
- Vivol
- Zetran
- Zipan
Eurosan Brand Names Mixture
- No information avaliable
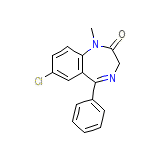
Eurosan Chemical_Formula
C16H13ClN2O
Eurosan RX_link
http://www.rxlist.com/cgi/generic/diazepam.htm
Eurosan fda sheet
Eurosan msds (material safety sheet)
Eurosan Synthesis Reference
Reeder, Sternbach; U.S. Pat. 3,371,085 (1968)
Eurosan Molecular Weight
284.74 g/mol
Eurosan Melting Point
125-126oC
Eurosan H2O Solubility
Slightly soluble (50 mg/L)
Eurosan State
solid
Eurosan LogP
2.988
Eurosan Dosage Forms
Tablets; Injectable solution
Eurosan Indication
Used in the treatment of severe anxiety disorders, as a hypnotic in the short-term management of insomnia, as a sedative and premedicant, as an anticonvulsant, and in the management of alcohol withdrawal syndrome.
Eurosan Pharmacology
Diazepam, a benzodiazepine, generates the same active metabolite as chlordiazepoxide and clorazepate. In animals, diazepam appears to act on parts of the limbic system, the thalamus and hypothalamus, and induces calming effects. Diazepam, unlike chlorpromazine and reserpine, has no demonstrable peripheral autonomic blocking action, nor does it produce extrapyramidal side effects; however, animals treated with diazepam do have a transient ataxia at higher doses. Diazepam was found to have transient cardiovascular depressor effects in dogs. Long-term experiments in rats revealed no disturbances of endocrine function. Injections into animals have produced localized irritation of tissue surrounding injection sites and some thickening of veins after intravenous use.
Eurosan Absorption
Essentially complete, with a bioavailability of 93%.
Eurosan side effects and Toxicity
Symptoms of overdose include somnolence, confusion, coma, and diminished reflexes. Respiration, pulse and blood pressure should be monitored.
Eurosan Patient Information
Eurosan Organisms Affected
Humans and other mammals