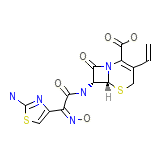
Cefdinir (JP14/USAN)




Cefdinir (JP14/USAN) Brand names, Cefdinir (JP14/USAN) Analogs
Cefdinir (JP14/USAN) Brand Names Mixture
- No information avaliable
Cefdinir (JP14/USAN) Chemical_Formula
C14H13N5O5S2
Cefdinir (JP14/USAN) RX_link
http://www.rxlist.com/cgi/generic2/cefdin.htm
Cefdinir (JP14/USAN) fda sheet
Cefdinir (JP14/USAN) msds (material safety sheet)
Cefdinir (JP14/USAN) Synthesis Reference
No information avaliable
Cefdinir (JP14/USAN) Molecular Weight
395.416 g/mol
Cefdinir (JP14/USAN) Melting Point
No information avaliable
Cefdinir (JP14/USAN) H2O Solubility
No information avaliable
Cefdinir (JP14/USAN) State
Solid
Cefdinir (JP14/USAN) LogP
0.657
Cefdinir (JP14/USAN) Dosage Forms
Capsules for oral administration (300mg); Oral suspension (125mg per 5mL solution)
Cefdinir (JP14/USAN) Indication
For the treatment of the respiratory, skin, soft tissue, and ENT infections caused by H. influenzae (including b-lactamase producing strains), H. parainfluenzae (including b-lactamase producing strains), S. pneumoniae (penicillin-susceptible strains), S. pyogenes, S. aureus (including b-lactamase producing strains), and M. catarrhalis.
Cefdinir (JP14/USAN) Pharmacology
Cefdinir is a third generation cephalosporin with a broad spectrum of activity against enteric gram-negative rods. Cefdinir is stable in the presence of some, but not all, b-lactamase enzymes. As a result, many organisms resistant to penicillins and some cephalosporins are susceptible to cefdinir. Cephalosporins work the same way as penicillins: they interfere with the peptidoglycan synthesis of the bacterial wall by inhibiting the final transpeptidation needed for the cross-links. This effect is bactericidal.
Cefdinir (JP14/USAN) Absorption
Maximal plasma cefdinir concentrations occur 2 to 4 hours postdose following capsule or suspension administration. Estimated bioavailability of cefdinir capsules is 21% following administration of a 300 mg capsule dose, and 16% following administration of a 600 mg capsule dose. Estimated absolute bioavailability of cefdinir suspension is 25%. Absorption is reduced by approximately 15% when administered with a high fat meal.
Cefdinir (JP14/USAN) side effects and Toxicity
Information on cefdinir overdosage in humans is not available. In acute rodent toxicity studies, a single oral 5600-mg/kg dose produced no adverse effects. Toxic signs and symptoms following overdosage with other b-lactam antibiotics have included nausea, vomiting, epigastric distress, diarrhea, and convulsions.
Cefdinir (JP14/USAN) Patient Information
Cefdinir (JP14/USAN) Organisms Affected
Enteric gram-negative rods