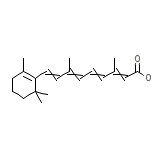
Eudyna




Eudyna Brand names, Eudyna Analogs
- Aberel
- Accutane
- Airol
- Aknefug
- Aknoten
- All Trans Retinoic Acid
- All Trans-Retinoic Acid
- Amnesteem
- Avita
- Beta-Retinoic Acid
- Claravis
- Dermairol
- Eudyna
- Isotretinoin
- Lsotretinoin
- Panretin
- Renova
- Retin-A
- Retin-a Micro
- Retinoate
- Retinoic Acid
- Retinoic Acid, All Trans Isomer
- Retionic Acid
- Retisol-A
- Solage
- Sotret
- Stieva-A
- Stieva-a Forte
- Trans-Retinoic Acid
- Tretin M
- Tri-Luma
- Vesanoid
- Vitamin a Acid
- Vitinoin
- ??-Retinoic Acid
Eudyna Brand Names Mixture
- No information avaliable
Eudyna Chemical_Formula
C20H28O2
Eudyna RX_link
http://www.rxlist.com/cgi/generic/tretinoin.htm
Eudyna fda sheet
Eudyna msds (material safety sheet)
Eudyna Synthesis Reference
Matsui et al.; J.Vitaminol.; 4; 190, 192(1958)
Eudyna Molecular Weight
300.435 g/mol
Eudyna Melting Point
181 oC
Eudyna H2O Solubility
<0.1 g/100 mL
Eudyna State
Solid
Eudyna LogP
5.067
Eudyna Dosage Forms
Capsule; Cream; Gel; Liquid
Eudyna Indication
For the the induction of remission in patients with acute promyelocytic leukemia (APL), French-American-British (FAB) classification M3 (including the M3 variant); For the topical treatment of acne vulgaris, flat warts and other skin conditions (psoriasis, ichthyosis congenita, icthyosis vulgaris, lamellar icthyosis, keratosis palmaris et plantaris, epidermolytic hyperkeratosis, senile comedones, senile keratosis, keratosis follicularis (Darier's disease), and basal cell carcinomas.); For palliative therapy to improve fine wrinkling, mottled hyperpigmentation, roughness associated with photodamage.
Eudyna Pharmacology
Tretinoin, also known as all-trans-retinoic acid (ATRA), is a naturally occurring derivative of vitamin A (retinol). Retinoids such as tretinoin are important regulators of cell reproduction, proliferation, and differentiation and are used to treat acne and photodamaged skin and to manage keratinization disorders such as ichthyosis and keratosis follicularis. Tretinoin also represents the class of anticancer drugs called differentiating agents and is used in the treatment of acute promyelocytic leukemia (APL).
Eudyna Absorption
1-31% (topical);
Eudyna side effects and Toxicity
No information avaliable
Eudyna Patient Information
No information avaliable
Eudyna Organisms Affected
Humans and other mammals