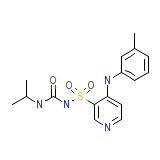
Torasemidum (INN-Latin)




Torasemidum (INN-Latin) Brand names, Torasemidum (INN-Latin) Analogs
Torasemidum (INN-Latin) Brand Names Mixture
- No information avaliable
Torasemidum (INN-Latin) Chemical_Formula
C16H20N4O3S
Torasemidum (INN-Latin) RX_link
http://www.rxlist.com/cgi/generic/demadex.htm
Torasemidum (INN-Latin) fda sheet
Torasemidum (INN-Latin) msds (material safety sheet)
Torasemidum (INN-Latin) Synthesis Reference
No information avaliable
Torasemidum (INN-Latin) Molecular Weight
348.421 g/mol
Torasemidum (INN-Latin) Melting Point
163-164 oC (decomposition)
Torasemidum (INN-Latin) H2O Solubility
Soluble
Torasemidum (INN-Latin) State
Solid
Torasemidum (INN-Latin) LogP
2.404
Torasemidum (INN-Latin) Dosage Forms
Liquid; Tablet
Torasemidum (INN-Latin) Indication
For the treatment of hypertension, edema induced by congestive heart failure or renal disease, and ascites associated with hepatic disease.
Torasemidum (INN-Latin) Pharmacology
Torasemide, a monosulfonamyl loop diuretic, differs form other thiazide diuretics in that a double ring system is incorporated into its structure. Torasemide is used alone or with atenolol in the management of hypertension and edema. Micropuncture studies in animals have shown that torsemide acts from within the lumen of the thick ascending portion of the loop of Henle, where it inhibits the Na+/K+/2Cl--carrier system.
Torasemidum (INN-Latin) Absorption
Bioavailability is approximately 80%. Serum concentration peaks after approximately 1 hour.
Torasemidum (INN-Latin) side effects and Toxicity
LD50 = 5 g/kg (rat, oral), 500 mg/kg (rat, intravenous). Side effects include dizziness, headache, nausea and vomiting.
Torasemidum (INN-Latin) Patient Information
No information avaliable
Torasemidum (INN-Latin) Organisms Affected
Humans and other mammals