Cefspan




Cefspan Brand names, Cefspan Analogs
Cefspan Brand Names Mixture
- No information avaliable
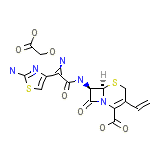
Cefspan Chemical_Formula
C16H15N5O7S2
Cefspan RX_link
http://www.rxlist.com/cgi/generic/cefixime.htm
Cefspan fda sheet
Cefspan msds (material safety sheet)
Cefspan Synthesis Reference
T. Takaya et al., U.S. Pat. 4,409,214 (1981)
Cefspan Molecular Weight
453.452 g/mol
Cefspan Melting Point
218-225 oC
Cefspan H2O Solubility
55.11 mg/L
Cefspan State
Solid
Cefspan LogP
-0.559
Cefspan Dosage Forms
Oral suspension (100 mg/5 mL); Tablet (200 mg and 400 mg); Powder for solution; Powder for suspension
Cefspan Indication
For use in the treatment of the following infections when caused by susceptible strains of the designated microorganisms: (1) uncomplicated urinary tract infections caused by Escherichia coli and Proteus mirabilis, (2) otitis media caused by Haemophilus influenzae (beta-lactamase positive and negative strains), Moraxella catarrhalis (most of which are beta-lactamase positive), and S. pyogenes, (3) pharyngitis and tonsillitis caused by S. pyogenes, (4) acute bronchitis and acute exacerbations of chronic bronchitis caused by Streptococcus pneumoniae and Haemophilus influenzae (beta-lactamase positive and negative strains), and (5) uncomplicated gonorrhea (cervical/urethral) caused by Neisseria gonorrhoeae (penicillinase- and non-penicillinase-producing strains).
Cefspan Pharmacology
Cefixime, an antibiotic, is a third-generation cephalosporin like ceftriaxone and cefotaxime. Cefixime is highly stable in the presence of beta-lactamase enzymes. As a result, many organisms resistant to penicillins and some cephalosporins due to the presence of beta-lactamases, may be susceptible to cefixime. The antibacterial effect of cefixime results from inhibition of mucopeptide synthesis in the bacterial cell wall.
Cefspan Absorption
About 40%-50% absorbed orally whether administered with or without food, however, time to maximal absorption is increased approximately 0.8 hours when administered with food.
Cefspan side effects and Toxicity
Symptoms of overdose include blood in the urine, diarrhea, nausea, upper abdominal pain, and vomiting.
Cefspan Patient Information
Cefspan Organisms Affected
Enteric bacteria and other eubacteria