Avalide




Avalide Brand names, Avalide Analogs
Avalide Brand Names Mixture
- Avalide 150/12.5 mg (Hydrochlorothiazide + Irbesartan)
- Avalide 300/12.5 mg (Hydrochlorothiazide + Irbesartan)
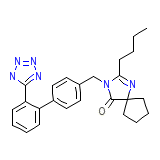
Avalide Chemical_Formula
C25H28N6O
Avalide RX_link
http://www.rxlist.com/cgi/generic/irbesart.htm
Avalide fda sheet
Avalide msds (material safety sheet)
Avalide Synthesis Reference
C. Bernhart et al.; U.S. Pat. 5,270,317 (1993)
Avalide Molecular Weight
428.53 g/mol
Avalide Melting Point
180-181 oC
Avalide H2O Solubility
No information avaliable
Avalide State
Solid
Avalide LogP
5.379
Avalide Dosage Forms
Tablet (oral)
Avalide Indication
For the treatment of hypertension, and for the treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria (>300 mg/day) in patients with type 2 diabetes and hypertension.
Avalide Pharmacology
Irbesartan is a specific competitive antagonist of AT1 receptors with a much greater affinity (more than 8500-fold) for the AT1 receptor than for the AT2 receptor and no agonist activity.
Avalide Absorption
Rapid and complete with an average absolute bioavailability of 60-80%. Food has no affect on bioavailability.
Avalide side effects and Toxicity
Hypotension and tachycardia; bradycardia might also occur from overdose, LD50=mg/kg(orally in rat)
Avalide Patient Information
Pregnancy: Female patients of childbearing age should be told about the consequences of second- and third-trimester exposure to drugs that act on the renin-angiotensin system, and they should also be told that these consequences do not appear to have resulted from intrauterine drug exposure that has been limited to the first trimester. These patients should be asked to report pregnancies to their physicians as soon as possible.
Avalide Organisms Affected
Humans and other mammals