Amifostine Ethiofos




Amifostine Ethiofos Brand names, Amifostine Ethiofos Analogs
Amifostine Ethiofos Brand Names Mixture
- No information avaliable
Amifostine Ethiofos Chemical_Formula
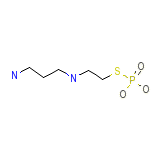
C5H15N2O3PS
Amifostine Ethiofos RX_link
http://www.rxlist.com/cgi/generic/amifostine.htm
Amifostine Ethiofos fda sheet
Amifostine Ethiofos msds (material safety sheet)
Amifostine Ethiofos Synthesis Reference
No information avaliable
Amifostine Ethiofos Molecular Weight
214.224 g/mol
Amifostine Ethiofos Melting Point
No information avaliable
Amifostine Ethiofos H2O Solubility
1000 mg/mL
Amifostine Ethiofos State
Solid
Amifostine Ethiofos LogP
-1.043
Amifostine Ethiofos Dosage Forms
Powder for solution
Amifostine Ethiofos Indication
For reduction in the cumulative renal toxicity in patients with ovarian cancer (using cisplatin) and moderate to severe xerostomia in patients undergoing post-operative radiation treatment for head and neck cancer.
Amifostine Ethiofos Pharmacology
Amifostine is an organic thiophosphate cytoprotective agent indicated to reduce the cumulative renal toxicity associated with repeated administration of cisplatin in patients with advanced ovarian cancer or non-small cell lung cancer and also to reduce the incidence of moderate to severe xerostomia in patients undergoing post-operative radiation treatment for head and neck cancer. Amifostine is a prodrug that is dephosphorylated by alkaline phosphatase in tissues to a pharmacologically active free thiol metabolite, believed to be responsible for the reduction of the cumulative renal toxicity of cisplatin and for the reduction of the toxic effects of radiation on normal oral tissues. Healthy cells are preferentially protected because amifostine and metabolites are present in healthy cells at 100-fold greater concentrations than in tumour cells.
Amifostine Ethiofos Absorption
No information avaliable
Amifostine Ethiofos side effects and Toxicity
Rat LD50: 826 mg/kg
Amifostine Ethiofos Patient Information
No information avaliable
Amifostine Ethiofos Organisms Affected
Humans and other mammals