Abbott 45975




Abbott 45975 Brand names, Abbott 45975 Analogs
Abbott 45975 Brand Names Mixture
- No information avaliable
Abbott 45975 Chemical_Formula
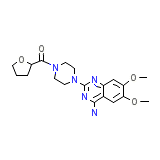
C19H25N5O4
Abbott 45975 RX_link
http://www.rxlist.com/cgi/generic/teraz.htm
Abbott 45975 fda sheet
Abbott 45975 msds (material safety sheet)
Abbott 45975 Synthesis Reference
M. Winn et al., U.S. Pat. 4,026,894 (1977)
Abbott 45975 Molecular Weight
387.433 g/mol
Abbott 45975 Melting Point
273 oC
Abbott 45975 H2O Solubility
29.7mg/mL
Abbott 45975 State
Solid
Abbott 45975 LogP
0.875
Abbott 45975 Dosage Forms
Tablet
Abbott 45975 Indication
For the treatment of symptomatic benign prostatic hyperplasia (BPH) and also for hypertension.
Abbott 45975 Pharmacology
Terazosin, classified as a quinazoline, is similar to doxazosin and prazosin. As an alpha-adrenergic blocking agent, terazosin is used to treat hypertension and benign prostatic hypertrophy (BPH). Terazosin produces vasodilation and reduces peripheral resistance but in general has slight effect on cardiac output. Antihypertensive effect with chronic dosing is usually not accompanied by reflex tachycardia.
Abbott 45975 Absorption
Essentially completely absorbed in man (90% bioavailability).
Abbott 45975 side effects and Toxicity
LD50=259.3mg/kg (i.v. in mice)
Abbott 45975 Patient Information
Abbott 45975 Organisms Affected
Humans and other mammals