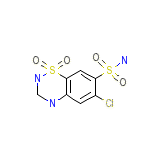
Chlorsulfonamidodihydrobenzothiadiazine Dioxide




Categoria
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Marchi, Chlorsulfonamidodihydrobenzothiadiazine Dioxide Analoghi
- Acuretic
- Aldactazide
- Aldoril
- Apresazide
- Aquarills
- Aquarius
- Bremil
- Caplaril
- Capozide
- Chlorosulthiadil
- Chlorothiazide
- Chlorsulfonamidodihydrobenzothiadiazine Dioxide
- Chlorzide
- Cidrex
- Dichlorosal
- Dichlorotride
- Dichlotiazid
- Dichlotride
- Diclotride
- Dicyclotride
- Dihydrochlorothiazid
- Dihydrochlorothiazide
- Dihydrochlorothiazidum
- Dihydrochlorurit
- Dihydrochlorurite
- Dihydroxychlorothiazidum
- Direma
- Disalunil
- Diu-Melusin
- Diuril
- Drenol
- Dyazide
- Esidrex
- Esidrix
- Esimil
- Fluvin
- HCTZ
- HCZ
- Hidril
- Hidrochlortiazid
- Hidroronol
- Hidrotiazida
- Hydril
- Hydro-Aquil
- Hydro-D
- Hydro-Diuril
- Hydrochlorothiazid
- Hydrochlorothiazide Intensol
- Hydrochlorthiazide
- Hydrodiuretic
- Hydrodiuril
- Hydropres
- Hydrosaluric
- Hydrothide
- Hydrozide
- Hypothiazid
- Hypothiazide
- Hyzaar
- Idrotiazide
- Inderide
- Ivaugan
- Jen-Diril
- Lopressor Hct
- Lotensin Hct
- Maschitt
- Maxzide
- Megadiuril
- Microzide
- Moduretic
- Nefrix
- Neo-Codema
- Neoflumen
- Newtolide
- Oretic
- Palonyl
- Panurin
- Perovex
- Primogyn
- Prinzide
- Ro-Hydrazide
- Servithiazid
- Thiaretic
- Thiuretic
- Thlaretic
- Timolide
- Unipres
- Urodiazin
- Vaseretic
- Vetidrex
- Ziac
- Zide
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Marchi miscela
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Formula chimica
C7H8ClN3O4S2
Chlorsulfonamidodihydrobenzothiadiazine Dioxide RX link
http://www.rxlist.com/cgi/generic/hctz.htm
Chlorsulfonamidodihydrobenzothiadiazine Dioxide FDA foglio
Chlorsulfonamidodihydrobenzothiadiazine Dioxide DMS (foglio di materiale di sicurezza)
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Sintesi di riferimento
Werner et al. J. Am. Chem. Soc. 82, 1161 (1960)
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Peso molecolare
297.741 g/mol
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Temperatura di fusione
274 oC
Chlorsulfonamidodihydrobenzothiadiazine Dioxide H2O Solubilita
0,7 mg / ml
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Stato
Solid
Chlorsulfonamidodihydrobenzothiadiazine Dioxide LogP
-0.268
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Forme di dosaggio
Tablet (orale)
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Indicazione
Per il trattamento della pressione alta e la gestione di edema.
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Farmacologia
I tiazidici, come l'idroclorotiazide favorire la perdita di acqua dal corpo (diuretici). Essi inibiscono la Na + / Cl-riassorbimento da parte del tubulo contorto distale nei reni. I diuretici tiazidici anche causare la perdita di potassio e un aumento di livelli sierici di acido urico. I diuretici tiazidici sono spesso usati per trattare l'ipertensione, ma i loro effetti ipotensivi non sono necessariamente a causa della loro attività diuretica. I diuretici tiazidici hanno dimostrato di prevenire ipertensione correlata morbilità e mortalità anche se il meccanismo non è completamente nota. I diuretici tiazidici provocare vasodilatazione mediante l'attivazione canali del potassio calcio-attivata (conduttanza di grandi dimensioni) in vascolare muscolatura liscia e inibendo vari carbonica anhydrases nel tessuto vascolare.
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Assorbimento
50-60%
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Tossicita
I segni e sintomi più comuni osservati sono quelli causati dalla deplezione elettrolitica (ipopotassiemia, ipocloremia, iposodiemia) e disidratazione conseguente a diuresi eccessiva. Se il digitale ha anche , l'ipokalemia può accentuare le aritmie cardiache. La DL50 orale di idroclorotiazide è superiore a 10 g / kg nel topo e ratto.
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Informazioni paziente
General
All patients receiving diuretic therapy should be observed for evidence of fluid or electrolyte
imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine
electrolyte determinations are particularly important when the patient is vomiting excessively or
receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance,
irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness,
restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension,
oliguria, tachycardia, and gastrointestinal disturbance such as nausea or vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present or
after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia.
Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of
the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Hypokalemia may be avoided or treated by use of potassium sparing diuretics or potassium
supplements such as foods with a high potassium content.
Although any chloride deficit is generally mild and usually does not require specific treatment
except under extraordinary circumstances (as in liver disease or renal disease), chloride
replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is
water restriction, rather than administration of salt, except in rare instances when the
hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the
therapy of choice.
Hyperuricemia may occur or acute gout may be precipitated in certain patients receiving thiazides.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required.
Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest
during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation
of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be
evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for
parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Laboratory Tests
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be done
at appropriate intervals.
All patients receiving diuretic therapy should be observed for evidence of fluid or electrolyte
imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine
electrolyte determinations are particularly important when the patient is vomiting excessively or
receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance,
irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness,
restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension,
oliguria, tachycardia, and gastrointestinal disturbance such as nausea or vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present or
after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia.
Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of
the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Hypokalemia may be avoided or treated by use of potassium sparing diuretics or potassium
supplements such as foods with a high potassium content.
Although any chloride deficit is generally mild and usually does not require specific treatment
except under extraordinary circumstances (as in liver disease or renal disease), chloride
replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is
water restriction, rather than administration of salt, except in rare instances when the
hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the
therapy of choice.
Hyperuricemia may occur or acute gout may be precipitated in certain patients receiving thiazides.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required.
Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest
during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation
of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be
evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for
parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Laboratory Tests
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be done
at appropriate intervals.
Chlorsulfonamidodihydrobenzothiadiazine Dioxide Atto interessato organismi
Gli esseri umani e altri mammiferi