Salofalk




Salofalk Brand names, Salofalk Analogs
Salofalk Brand Names Mixture
- No information avaliable
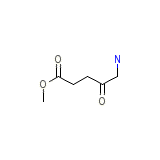
Salofalk Chemical_Formula
C6H11NO3
Salofalk RX_link
No information avaliable
Salofalk fda sheet
Salofalk msds (material safety sheet)
Salofalk Synthesis Reference
No information avaliable
Salofalk Molecular Weight
145.156 g/mol
Salofalk Melting Point
No information avaliable
Salofalk H2O Solubility
Freely soluble
Salofalk State
Solid
Salofalk LogP
-1.026
Salofalk Dosage Forms
Cream for topical administration (160 mg/g of methyl aminolevulinate equivalent to 16.0% of methyl aminolevulinate)
Salofalk Indication
For topical use, in combination with 570 to 670 nm wavelength red light illumination, in the treatment of non-hyperkeratotic actinic keratoses of the face and scalp in immunocompetent patients when used in conjunction with lesion preparation (debridement using a sharp dermal curette).
Salofalk Pharmacology
After topical application of methyl aminolevulinate, porphyrins will accumulate intracellularly in the treated skin lesions. The intracellular porphyrins (including PpIX) are photoactive, fluorescing compounds and, upon light activation in the presence of oxygen, singlet oxygen is formed which causes damage to cellular compartments, in particular the mitochondria. Light activation of accumulated porphyrins leads to a photochemical reaction and thereby phototoxicity to the light-exposed target cells.
Salofalk Absorption
In vitro, after 24 hours the mean cumulative absorption through human skin was 0.26% of the administered dose.
Salofalk side effects and Toxicity
The severity of local phototoxic reactions such as erythema, pain and burning sensation may increase in case of prolonged application time or very high light intensity.
Salofalk Patient Information
Salofalk Organisms Affected
Humans and other mammals