Colesevelam hydrochloride




Colesevelam hydrochloride Brand names, Colesevelam hydrochloride Analogs
Colesevelam hydrochloride Brand Names Mixture
- No information avaliable
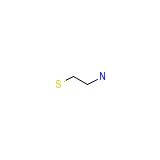
Colesevelam hydrochloride Chemical_Formula
C2H7NS
Colesevelam hydrochloride RX_link
No information avaliable
Colesevelam hydrochloride fda sheet
Colesevelam hydrochloride msds (material safety sheet)
Colesevelam hydrochloride Synthesis Reference
No information avaliable
Colesevelam hydrochloride Molecular Weight
77.1497 g/mol
Colesevelam hydrochloride Melting Point
98 oC
Colesevelam hydrochloride H2O Solubility
No information avaliable
Colesevelam hydrochloride State
Solid
Colesevelam hydrochloride LogP
-0.196
Colesevelam hydrochloride Dosage Forms
Capsule (50 mg, 150 mg)
Colesevelam hydrochloride Indication
Given intravenously or orally to treat radiation sickness. The bitartrate has been used for the oral treatment of nephropathic cystinosis.
Colesevelam hydrochloride Pharmacology
People born without the ability to metabolize the amino acid cystine suffer from cystinosis, a rare inherited disorder characterized by the deposition and accumulation of cystine crystals throughout the body. These crystals cause considerable damage, particularly in the kidney. Kidney failure can occur by the age of 10 in untreated patients. Cysteamine prevents the accumulation of cystine crystals and is prescribed to prevent further kidney damage. Cysteamine helps to convert cystine into less harmful chemical forms that can be removed from cells.
Colesevelam hydrochloride Absorption
No information avaliable
Colesevelam hydrochloride side effects and Toxicity
Symptoms of overdose may include convulsions (seizures), increased thirst and unusual tiredness or weakness.
Colesevelam hydrochloride Patient Information
No information avaliable
Colesevelam hydrochloride Organisms Affected
Humans and other mammals