Ziba-rx




Ziba-rx Brand names, Ziba-rx Analogs
Ziba-rx Brand Names Mixture
- No information avaliable
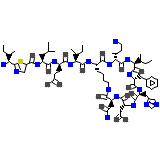
Ziba-rx Chemical_Formula
C66H103N17O16S
Ziba-rx RX_link
http://www.rxlist.com/cgi/generic3/bacit.htm
Ziba-rx fda sheet
Ziba-rx msds (material safety sheet)
Ziba-rx Synthesis Reference
No information avaliable
Ziba-rx Molecular Weight
1422.69 g/mol
Ziba-rx Melting Point
221 - 225 oC
Ziba-rx H2O Solubility
Freely soluble
Ziba-rx State
Solid
Ziba-rx LogP
-1.025
Ziba-rx Dosage Forms
Drug premix; Ointment; Powder; Powder for solution
Ziba-rx Indication
For the treatment of infants with pneumonia and empyema caused by staphylococci shown to be susceptible to the drug. Also used in ointment form for topical treatment of a variety of localized skin and eye infections, as well as for the prevention of wound infections.
Ziba-rx Pharmacology
Bacitracin is a mixture of related cyclic polypeptides produced by organisms of the licheniformis group of Bacillus subtilis var Tracy. As a polypeptide, toxic, and difficult to use chemical, Bacitracin doesn't work well orally, however is very effective topically. Bacitracin exerts pronounced antibacterial action in vitro against a variety of gram-positive and a few gram-negative organisms. However, among systemic diseases, only staphylococcal infections qualify for consideration of bacitracin therapy.
Ziba-rx Absorption
Absorption of bacitracin following intramuscular injection is rapid and complete. Absorption from the gastrointestinal tract following oral administration is not appreciable. Absorption following topical application is negligible.
Ziba-rx side effects and Toxicity
Oral, mouse: LD50 = >3750 mg/kg.
Ziba-rx Patient Information
Ziba-rx Organisms Affected
Enteric bacteria and other eubacteria