Singulair




Categoria
Singulair Marchi, Singulair Analoghi
Singulair Marchi miscela
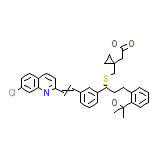
Singulair Formula chimica
C35H36ClNO3S
Singulair RX link
http://www.rxlist.com/cgi/generic3/monteluk.htm
Singulair FDA foglio
Singulair DMS (foglio di materiale di sicurezza)
Singulair Sintesi di riferimento
Belley ML et al. Eur. Pat. Appl. 480,717 (1992)
Singulair Peso molecolare
586.184 g/mol
Singulair Temperatura di fusione
No information avaliable
Singulair H2O Solubilita
Nessuna informazione disponibile
Singulair Stato
Solid
Singulair LogP
8.488
Singulair Forme di dosaggio
Tablet (orale)
Singulair Indicazione
Per il trattamento di asma
Singulair Farmacologia
Montelukast, zafirlukast come, è un antagonista del recettore dei leucotrieni utilizzato come alternativa ai farmaci anti-infiammatori nella gestione e nel trattamento cronico dell'asma e di esercizio broncospasmo indotto (BEI). A differenza di zafirlukast, montelukast non inibisce il CYP2C9 o CYP3A4 ed è, quindi, non dovrebbe influenzare la clearance epatica dei farmaci metabolizzati da questi enzimi.
Singulair Assorbimento
Rapidamente assorbito dopo somministrazione orale (biodisponibilità è del 64%)
Singulair Tossicita
Gli effetti collaterali includono mal di testa, dolore addominale o mal di stomaco, tosse, mal di denti, vertigini, febbre, bruciore di stomaco, eruzioni cutanee, naso chiuso, debolezza o stanchezza inusuale.
Singulair Informazioni paziente
General
- Patients should be advised to take montelukast daily as prescribed, even when they are asymptomatic, as well as during periods of worsening asthma, and to contact their physicians if their asthma is not well controlled.
- Patients should be advised that oral tablets of montelukast are not for the treatment of acute asthma attacks. They should have appropriate short-acting inhaled b-agonist medication available to treat asthma exacerbations.
- Patients should be advised that, while using montelukast, medical attention should be sought if short-acting inhaled bronchodilators are needed more often than usual, or if more than the maximum number of inhalations of short-acting bronchodilator treatment prescribed for 24-hour period are needed.
- Patients receiving montelukast should be instructed not to decrease the dose or stop taking any other antiasthma medications unless instructed by a physician.
- Patients who have exacerbations of asthma after exercise should be instructed to continue to use their usual regimen of inhaled b-agonists as prophylaxis unless otherwise instructed by their physician. All patients should have available for rescue a short-acting inhaled b-agonist.
- Patients with known aspirin sensitivity should be advised to continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast.
Phenylketonurics: Phenylketonuric patients should be informed that the chewable tablet contains phenylalanine (a component of aspartame) 0.842 mg per 5-mg chewable tablet.
Singulair Atto interessato organismi
Gli esseri umani e altri mammiferi