Orfadin




Orfadin Brand names, Orfadin Analogs
Orfadin Brand Names Mixture
- No information avaliable
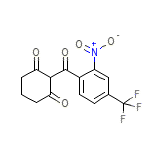
Orfadin Chemical_Formula
C14H10F3NO5
Orfadin RX_link
http://www.rxlist.com/cgi/generic/orfadin.htm
Orfadin fda sheet
Orfadin msds (material safety sheet)
Orfadin Synthesis Reference
No information avaliable
Orfadin Molecular Weight
329.228 g/mol
Orfadin Melting Point
No information avaliable
Orfadin H2O Solubility
No information avaliable
Orfadin State
No information avaliable
Orfadin LogP
2.105
Orfadin Dosage Forms
Capsules; Liquid
Orfadin Indication
Used as an adjunct to dietary restriction of tyrosine and phenylalanine in the treatment of hereditary tyrosinemia type 1.
Orfadin Pharmacology
Hereditary tyrosinemia type 1 occurs due to a deficiency in fumarylacetoacetase (FAH), the final enzyme in the tyrosine catabolic pathway. Nitisinone inhibits catabolism of tyrosine by preventing the catabolic intermediates. In patients with HT-1, these catabolic intermediates are converted to the toxic metabolites succinylacetone and succinylacetoacetate, which are responsible for the observed liver and kidney toxicity. Succinylacetone can also inhibit the porphyrin synthesis pathway leading to the accumulation of 5-aminolevulinate, a neurotoxin responsible for the porphyric crises characteristic of HT-1.
Orfadin Absorption
The capsule and liquid formulations are bioequivalent in both the plasma concentration-time curve and maximum plasma concentration (Cmax).
Orfadin side effects and Toxicity
Side effects include elevated plasma levels of this amino acid, hepatic and liver failure.
Orfadin Patient Information
Orfadin Organisms Affected
Humans and other mammals