Cranoc




Cranoc Brand names, Cranoc Analogs
Cranoc Brand Names Mixture
- No information avaliable
Cranoc Chemical_Formula
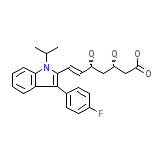
C24H26FNO4
Cranoc RX_link
http://www.rxlist.com/cgi/generic2/fluvastatinxl.htm
Cranoc fda sheet
Cranoc msds (material safety sheet)
Cranoc Synthesis Reference
F. G. Kathawala, U.S. Pat. 4,739,073 (1988)
Cranoc Molecular Weight
411.466 g/mol
Cranoc Melting Point
194-197oC
Cranoc H2O Solubility
0.46 mg/L
Cranoc State
Solid
Cranoc LogP
4.98
Cranoc Dosage Forms
Capsules; Tablets (extended-release)
Cranoc Indication
For management as an adjunct to diet to reduce elevated total-C, LDL-C, apo B, and TG levels in patients with primary hypercholesterolemia and mixed dyslipidemia
Cranoc Pharmacology
Fluvastatin, the first synthetically-prepared HMG-CoA reductase inhibitor, is used as an antilipemic to lower cholesterol and triglyceride levels associated with primary hypercholesterolemia and mixed dyslipidemia (Fredrickson types IIa and IIb) and to slow the progression of coronary atherosclerosis in patients with coronary artery disease. Although similar to lovastatin, simvastatin, and pravastatin, Fluvastatin has a shorter half-life, no active metabolites, extensive protein binding, and minimal CSF penetration.
Cranoc Absorption
24% (range 9%-50%).
Cranoc side effects and Toxicity
Variety of GI complaints and an increase in transaminase values (i.e., SGOT and SGPT).
Cranoc Patient Information
PATIENT INFORMATION
Patients should be advised to report promptly unexplained muscle pain, tenderness or
weakness, particularly if accompanied by malaise or fever.
Women should be informed that if they become pregnant while receiving Lescol or Lescol
XL the drug should be discontinued immediately to avoid possible harmful effects on a
developing fetus from a relative deficit of cholesterol and biological products derived
from cholesterol. In addition, Lescol or Lescol XL should not be taken during nursing.
Patients should be advised to report promptly unexplained muscle pain, tenderness or
weakness, particularly if accompanied by malaise or fever.
Women should be informed that if they become pregnant while receiving Lescol or Lescol
XL the drug should be discontinued immediately to avoid possible harmful effects on a
developing fetus from a relative deficit of cholesterol and biological products derived
from cholesterol. In addition, Lescol or Lescol XL should not be taken during nursing.
Cranoc Organisms Affected
Humans and other mammals