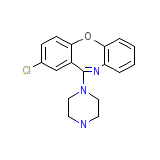
Asendis




Asendis Brand names, Asendis Analogs
Asendis Brand Names Mixture
- No information avaliable
Asendis Chemical_Formula
C17H16ClN3O
Asendis RX_link
http://www.rxlist.com/cgi/generic/amoxapine.htm
Asendis fda sheet
Asendis msds (material safety sheet)
Asendis Synthesis Reference
No information avaliable
Asendis Molecular Weight
313.781 g/mol
Asendis Melting Point
175.5 oC
Asendis H2O Solubility
No information avaliable
Asendis State
Solid
Asendis LogP
2.848
Asendis Dosage Forms
Tablet (25 mg, 50 mg, 100 mg or 150 mg)
Asendis Indication
For the relief of symptoms of depression in patients with neurotic or reactive depressive disorders as well as endogenous and psychotic depressions. Also for depression accompanied by anxiety or agitation.
Asendis Pharmacology
Amoxapine is a tricyclic antidepressant of the dibenzoxazepine class, chemically distinct from the dibenzodiazepines, dibenzocycloheptenes, and dibenzoxepines. It has a mild sedative component to its action. The mechanism of its clinical action in man is not well understood. In animals, amoxapine reduced the uptake of nor-epinephirine and serotonin and blocked the response of dopamine receptors to dopamine Amoxapine is not a monoamine oxidase inhibitor. Clinical studies have demonstrated that amoxapine has a more rapid onset of action than either amitriptyline or imipramine
Asendis Absorption
Absorbed rapidly and reaches peak blood levels approximately 90 minutes after ingestion
Asendis side effects and Toxicity
Toxic manifestations of amoxapine overdosage differ significantly from those of other tricyclic antidepressants. Serious cardiovascular effects are seldom if ever observed. However, CNS effects, particularly grand mal convulsions, occur frequently, and treatment should be directed primarily toward prevention or control of seizures. Status epilepticus may develop and constitutes a neurologic emergency. Coma and acidosis are other serious complications of substantial amoxapine overdosage in some cases. Renal failure may develop two to five days after toxic overdose in patients who may appear otherwise recovered. Acute tubular necrosis with rhabdomuolysis and myolobinurla is the most common renal complication in such cases. This reaction probably occurs in less than 5% of overdose cases, and typically in those who have experienced multiple seizures.
Asendis Patient Information
Given the likelihood that some patients exposed chronically to neuroleptics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided.
Patients should be warned of the possibility of drowsiness that may impair performance of potentially hazardous tasks such as driving an automobile or operating machinery.
Asendis Organisms Affected
Humans and other mammals