Cefadroxil Micronised Powder




Categorie
Cefadroxil Micronised Powder Les marques, Cefadroxil Micronised Powder Analogs
Cefadroxil Micronised Powder Les marques melange
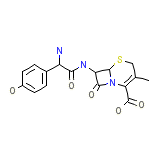
Cefadroxil Micronised Powder Formule chimique
C16H17N3O5S
Cefadroxil Micronised Powder RX lien
http://www.rxlist.com/cgi/generic/cefadrox.htm
Cefadroxil Micronised Powder FDA fiche
Cefadroxil Micronised Powder msds (fiche de securite des materiaux)
Cefadroxil Micronised Powder Synthese de reference
LB Crast, Jr., le brevet américain. 3,489,752 (1969)
Cefadroxil Micronised Powder Poids moleculaire
363.389 g/mol
Cefadroxil Micronised Powder Point de fusion
197oC
Cefadroxil Micronised Powder H2O Solubilite
1110 mg / L
Cefadroxil Micronised Powder Etat
Solid
Cefadroxil Micronised Powder LogP
-0.355
Cefadroxil Micronised Powder Formes pharmaceutiques
Capsule; Tablet; orale liquide
Cefadroxil Micronised Powder Indication
Pour le traitement des infections suivantes (peau, infections urinaires, ORL) causés par: S. pneumoniae, H. influenzae, staphylocoques, S. pyogenes (streptocoques du groupe A bêta-hémolytique), E. coli, P. mirabilis, Klebsiella sp, staphylocoques à coagulase négative et Streptococcus pyogenes
Cefadroxil Micronised Powder Pharmacologie
Cefadroxil, un antibiotique céphalosporine de première génération, est utilisé pour traiter les infections urinaires, infections structure de la peau et la peau, la pharyngite et l'amygdalite.
Cefadroxil Micronised Powder Absorption
Cefadroxil est bien absorbée sur l'administration orale, l'alimentation ne pas interférer avec l'absorption.
Cefadroxil Micronised Powder Toxicite
Nausées, vomissements, diarrhée, éruptions cutanées allergiques peuvent se produire
Cefadroxil Micronised Powder Information pour les patients
Patients should be counseled that intibacterial drugs including DURICEF should only be used to treat bacterial infections. They do not treat viral infections (eg. the common cold). When DURICEF is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelyhood that bacteria will develop resistance and will not be treatable by DURICEF or other antibacterial drugs in the future.
Cefadroxil Micronised Powder Organismes affectes
Bactéries entériques et d'autres eubactéries