Duricef




Duricef Brand names, Duricef Analogs
Duricef Brand Names Mixture
- None
Duricef Chemical_Formula
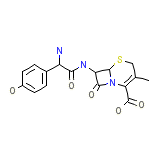
C16H17N3O5S
Duricef RX_link
http://www.rxlist.com/cgi/generic/cefadrox.htm
Duricef fda sheet
Duricef msds (material safety sheet)
Duricef Synthesis Reference
L. B. Crast, Jr., U.S. Pat. 3,489,752 (1969)
Duricef Molecular Weight
363.389 g/mol
Duricef Melting Point
197oC
Duricef H2O Solubility
1110 mg/L
Duricef State
Solid
Duricef LogP
-0.355
Duricef Dosage Forms
Capsule; Tablet; Oral liquid
Duricef Indication
For the treatment of the following infections (skin, UTI, ENT) caused by; S. pneumoniae, H. influenzae, staphylococci, S. pyogenes (group A beta-hemolytic streptococci), E. coli, P. mirabilis, Klebsiella sp, coagulase-negative staphylococci and Streptococcus pyogenes
Duricef Pharmacology
Cefadroxil, a first-generation cephalosporin antibiotic, is used to treat urinary tract infections, skin and skin structure infections, pharyngitis, and tonsillitis.
Duricef Absorption
Cefadroxil is well absorbed on oral administration; food does not interfere with its absorption.
Duricef side effects and Toxicity
Nausea, vomiting, diarrhoea, allergic rashes may occur
Duricef Patient Information
Patients should be counseled that intibacterial drugs including DURICEF should only be used to treat bacterial infections. They do not treat viral infections (eg. the common cold). When DURICEF is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelyhood that bacteria will develop resistance and will not be treatable by DURICEF or other antibacterial drugs in the future.
Duricef Organisms Affected
Enteric bacteria and other eubacteria