Semi-Euglucon




Semi-Euglucon Brand names, Semi-Euglucon Analogs
- Abbenclamide
- Adiab
- Apo-Glibenclamide
- Azuglucon
- Bastiverit
- Benclamin
- Betanase
- Betanese 5
- Calabren
- Cytagon
- Daonil
- Debtan
- Dia-basan
- Diabeta
- Diabeta (TN)
- Diabiphage
- Dibelet
- Duraglucon
- Euclamin
- Euglucan
- Euglucon
- Euglucon 5
- Euglykon
- GBN 5
- Gen-Glybe
- Gewaglucon
- Gilemal
- Glamide
- Glibadone
- Gliban
- Gliben
- Gliben-Puren N
- Glibenbeta
- Glibenclamid AL
- Glibenclamid Basics
- Glibenclamid Fabra
- Glibenclamid Genericon
- Glibenclamid Heumann
- Glibenclamid Riker M.
- Glibenclamid-Cophar
- Glibenclamid-Ratiopharm
- Glibenclamida [INN-Spanish]
- Glibenclamide
- Glibenclamide (JP14)
- Glibenclamidum [INN-Latin]
- Glibenil
- Glibens
- Glibesyn
- Glibet
- Glibetic
- Glibil
- Gliboral
- Glicem
- Glidiabet
- Glimel
- Glimide
- Glimidstata
- Glisulin
- Glitisol
- Glubate
- Gluben
- Gluco-Tablimen
- Glucobene
- Glucohexal
- Glucolon
- Glucomid
- Glucoremed
- Glucoven
- Glyben
- Glybenclamide
- Glybenzcyclamide
- Glyburide (USP)
- Glyburide (micronized)
- Glyburide [USAN]
- Glycolande
- Glycomin
- Glynase
- Hemi-Daonil
- Hexaglucon
- Humedia
- Lederglib
- Libanil
- Lisaglucon
- Malix
- Maninil
- Med-Glionil
- Melix
- Micronase
- Micronase (TN)
- Miglucan
- Nadib
- Neogluconin
- Norglicem 5
- Normoglucon
- Novo-Glyburide
- Orabetic
- Pira
- Praeciglucon
- PresTab
- Prodiabet
- Renabetic
- Semi-Euglucon
- Semi-Gliben-Puren N
- Semi-daonil
- Sugril
- Suraben
- Tiabet
- Yuglucon
Semi-Euglucon Brand Names Mixture
- No information avaliable
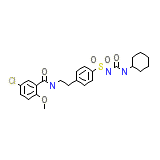
Semi-Euglucon Chemical_Formula
C23H28ClN3O5S
Semi-Euglucon RX_link
http://www.rxlist.com/cgi/generic/glybur.htm
Semi-Euglucon fda sheet
Semi-Euglucon msds (material safety sheet)
Semi-Euglucon Synthesis Reference
Weber et al., U.S. Pat. 3,454,635 (1969)
Semi-Euglucon Molecular Weight
494.004 g/mol
Semi-Euglucon Melting Point
169-170oC
Semi-Euglucon H2O Solubility
4 mg/L
Semi-Euglucon State
Solid
Semi-Euglucon LogP
4.85
Semi-Euglucon Dosage Forms
Tablet (1.25 mg, 2.5 mg, 5 mg)
Semi-Euglucon Indication
Indicated as an adjunct to diet to lower the blood glucose in patients with non-insulin-dependent diabetes mellitus (Type II) whose hyperglycemia cannot be satisfactorily controlled by diet alone.
Semi-Euglucon Pharmacology
Glyburide, a second-generation sulfonylurea antidiabetic agent, appears to lower the blood glucose acutely by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic islets. With chronic administration in Type II diabetic patients, the blood glucose lowering effect persists despite a gradual decline in the insulin secretory response to the drug. Extrapancreatic effects may be involved in the mechanism of action of oral sulfonyl-urea hypoglycemic drugs. The combination of glyburide and metformin may have a synergistic effect, since both agents act to improve glucose tolerance by different but complementary mechanisms. In addition to its blood glucose lowering actions, glyburide produces a mild diuresis by enhancement of renal free water clearance. Glyburide is twice as potent as the related second-generation agent glipizide.
Semi-Euglucon Absorption
Significant absorption within 1 hour and peak plasma levels are reached within 4 hours.
Semi-Euglucon side effects and Toxicity
Oral rat LD50: > 20,000 mg/kg. Oral mouse LD50: 3250 mg/kg.
Semi-Euglucon Patient Information
Patients should be informed of the potential risks and advantages of MICRONASE and of alternative modes of therapy. They also should be informed about the importance of adherence to dietary instructions, of a regular exercise program, and of regular testing of urine and/or blood glucose.
The risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and responsible family members. Primary and secondary failure also should be explained.
Semi-Euglucon Organisms Affected
Humans and other mammals