Dridol




Dridol Brand names, Dridol Analogs
Dridol Brand Names Mixture
- No information avaliable
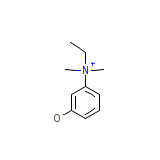
Dridol Chemical_Formula
C10H16NO+
Dridol RX_link
http://www.rxlist.com/cgi/generic3/edrophonium.htm
Dridol fda sheet
Dridol msds (material safety sheet)
Dridol Synthesis Reference
No information avaliable
Dridol Molecular Weight
166.24 g/mol
Dridol Melting Point
No information avaliable
Dridol H2O Solubility
Appreciable as liquid hydrochloride salt
Dridol State
Liquid
Dridol LogP
-2.95
Dridol Dosage Forms
Liquid for injection
Dridol Indication
For the differential diagnosis of myasthenia gravis and as an adjunct in the evaluation of treatment requirements in this disease. It may also be used for evaluating emergency treatment in myasthenic crises.
Dridol Pharmacology
Edrophonium is a short and rapid-acting anticholinesterase drug. Its effect is manifest within 30 to 60 seconds after injection and lasts an average of 10 minutes. Edrophonium's pharmacologic action is due primarily to the inhibition or inactivation of acetylcholinesterase at sites of cholinergic transmission. Muscarinic receptors are found throughout the body, especially on muscle. Stimulation of these receptors causes to muscle contraction. In myasthenia gravis the body's immune system destroys many of the muscarinic receptors, so that the muscle becomes less responsive to nervous stimulation. Edrophonium chloride increases the amount of acetylcholine at the nerve endings. Increased levels of acetyl choline allow the remaining receptors to function more efficiently.
Dridol Absorption
Rapidly absorbed.
Dridol side effects and Toxicity
With drugs of this type, muscarine-like symptoms (nausea, vomiting, diarrhea, sweating, increased bronchial and salivary secretions and bradycardia) often appear with overdosage (cholinergic crisis).
Dridol Patient Information
No information avaliable
Dridol Organisms Affected
Humans and other mammals