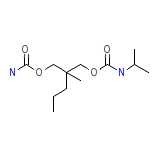
Carsodal




Carsodal Brand names, Carsodal Analogs
- Apesan
- Arusal
- Atonalyt
- Brianil
- Calenfa
- Caprodat
- Carisol
- Carisoma
- Carisoprodate
- Carisoprodatum
- Carlsodol
- Carlsoma
- Carlsoprol
- Carsodal
- Carsodol
- Coprobate
- Diolene
- Domarax
- Flexal
- Flexartal
- Flexartel
- Flibol E
- Isobamate
- Isomeprobamate
- Isopropyl Meprobamate
- Isoprotan
- Isoprotane
- Isoprothane
- Izoprotan
- Mediquil
- Meprobamate
- Meprocon
- Mioartrina
- Miolisodal
- Miolisodol
- Mioratrina
- Mioril
- Mioriodol
- Nospasm
- RELA
- Relasom
- Relax
- Sanoma
- Skutamil
- Soma
- Somadril
- Somalgit
- Somanil
- Stialgin
- Tonolyt Isopropyl Meprobamate
Carsodal Brand Names Mixture
- No information avaliable
Carsodal Chemical_Formula
Carsodal RX_link
Carsodal fda sheet
Carsodal msds (material safety sheet)
Carsodal Synthesis Reference
Carsodal Molecular Weight
Carsodal Melting Point
Carsodal H2O Solubility
Carsodal State
Carsodal LogP
Carsodal Dosage Forms
Carsodal Indication
Carsodal Pharmacology
Carsodal Absorption
Carsodal side effects and Toxicity
Carsodal Patient Information
Carisoprodol is a muscle relaxant used to relieve the pain and stiffness of muscle spasms and discomfort due to strain and sprain. Inform your physician if you are pregnant or nursing. Do not take this medication with a monoamine oxidase inhibitor. This medication may cause dizziness, drowsiness, or blurred vision; use caution while driving or operating hazardous machinery. Do not take any other sedating drugs or drink alcohol while taking carisoprodol. If dizziness occurs, avoid sudden changes in posture. Take this medication with food to avoid stomach upset. Notify your physician if you develop trouble breathing, unexplained fever, severe weakness, vision changes, swelling, or skin rash. Withdrawal symptoms may occur if therapy is suddenly stopped in a patient on long-term or high-dose therapy.