Carisoma




Carisoma Brand names, Carisoma Analogs
- Apesan
- Arusal
- Atonalyt
- Brianil
- Calenfa
- Caprodat
- Carisol
- Carisoma
- Carisoprodate
- Carisoprodatum
- Carlsodol
- Carlsoma
- Carlsoprol
- Carsodal
- Carsodol
- Coprobate
- Diolene
- Domarax
- Flexal
- Flexartal
- Flexartel
- Flibol E
- Isobamate
- Isomeprobamate
- Isopropyl Meprobamate
- Isoprotan
- Isoprotane
- Isoprothane
- Izoprotan
- Mediquil
- Meprobamate
- Meprocon
- Mioartrina
- Miolisodal
- Miolisodol
- Mioratrina
- Mioril
- Mioriodol
- Nospasm
- RELA
- Relasom
- Relax
- Sanoma
- Skutamil
- Soma
- Somadril
- Somalgit
- Somanil
- Stialgin
- Tonolyt Isopropyl Meprobamate
Carisoma Brand Names Mixture
- No information avaliable
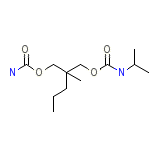
Carisoma Chemical_Formula
Carisoma RX_link
Carisoma fda sheet
Carisoma msds (material safety sheet)
Carisoma Synthesis Reference
Carisoma Molecular Weight
Carisoma Melting Point
Carisoma H2O Solubility
Carisoma State
Carisoma LogP
Carisoma Dosage Forms
Carisoma Indication
Carisoma Pharmacology
Carisoma Absorption
Carisoma side effects and Toxicity
Carisoma Patient Information
Carisoprodol is a muscle relaxant used to relieve the pain and stiffness of muscle spasms and discomfort due to strain and sprain. Inform your physician if you are pregnant or nursing. Do not take this medication with a monoamine oxidase inhibitor. This medication may cause dizziness, drowsiness, or blurred vision; use caution while driving or operating hazardous machinery. Do not take any other sedating drugs or drink alcohol while taking carisoprodol. If dizziness occurs, avoid sudden changes in posture. Take this medication with food to avoid stomach upset. Notify your physician if you develop trouble breathing, unexplained fever, severe weakness, vision changes, swelling, or skin rash. Withdrawal symptoms may occur if therapy is suddenly stopped in a patient on long-term or high-dose therapy.