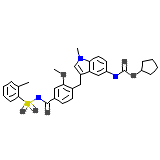
Zafirlukast




Categorie
Zafirlukast Les marques, Zafirlukast Analogs
Zafirlukast Les marques melange
Zafirlukast Formule chimique
Zafirlukast RX lien
Zafirlukast FDA fiche
Zafirlukast msds (fiche de securite des materiaux)
Zafirlukast Synthese de reference
Zafirlukast Poids moleculaire
Zafirlukast Point de fusion
Zafirlukast H2O Solubilite
Zafirlukast Etat
Zafirlukast LogP
Zafirlukast Formes pharmaceutiques
Zafirlukast Indication
Zafirlukast Pharmacologie
Zafirlukast Absorption
Zafirlukast Toxicite
Zafirlukast Information pour les patients
Patients should be told that a rare side effect of ACCOLATE is hepatic dysfunction, and to contact their physician immediately if they experience symptoms of hepatic dysfunction (eg. right upper quadrant abdominal pain, nausea, fatigue, lethargy, pruritus, jaundice, flu-like symptoms, and anorexia). Liver failure resulting in liver transplantation and death has occurred in patients taking zafirlukast .
ACCOLATE is indicated for the chronic treatment of asthma and should be taken regularly as prescribed, even during symptom-free periods. ACCOLATE is not a bronchodilator and should not be used to treat acute episodes of asthma. Patients receiving ACCOLATE should be instructed not to decrease the dose or stop taking any other antiasthma medications unless instructed by a physician. Women who are breast-feeding should be instructed not to take ACCOLATE. Alternative antiasthma medication should be considered in such patients.
The bioavailability of ACCOLATE may be decreased when taken with food. Patients should be instructed to take ACCOLATE at least 1 hour before or 2 hours after meals.
Eosinophilic Conditions: In rare cases, patients on ACCOLATE therapy may present with systemic eosinophilia, eosinophilic pneumonia, or clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic steroid therapy. These events usually, but not always, have been associated with the reduction of oral steroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between ACCOLATE and these underlying conditions has not been established .