Danoflox




Categorie
Danoflox Les marques, Danoflox Analogs
- Akilen
- Baccidal
- Bactocin
- Danoflox
- Effexin
- Exocin
- Exocine
- Flobacin
- Flodemex
- Flotavid
- Flovid
- Floxal
- Floxil
- Floxin
- Floxin Otic
- Floxstat
- Fugacin
- Inoflox
- Kinflocin
- Kinoxacin
- Liflox
- Loxinter
- Marfloxacin
- Medofloxine
- Mergexin
- Novecin
- Nufafloqo
- O-Flox
- Obide
- Occidal
- Ocuflox
- Ofcin
- Oflin
- Oflocee
- Oflocet
- Oflocin
- Oflodal
- Oflodex
- Oflodura
- Oflox
- Ofloxin
- Ofus
- Onexacin
- Operan
- Orocin
- Otonil
- Pharflox
- Praxin
- Puiritol
- Qinolon
- Qipro
- Quinolon
- Quotavil
- Rilox
- Sinflo
- Tabrin
- Taravid
- Tariflox
- Tarivid
- Tarivid Eye Ear
- Tarivid Otic
- Telbit
- Tructum
- Uro Tarivid
- Viotisone
- Zanocin
Danoflox Les marques melange
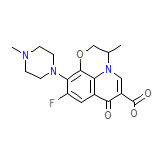
Danoflox Formule chimique
C18H20FN3O4
Danoflox RX lien
http://www.rxlist.com/cgi/generic/oflox.htm
Danoflox FDA fiche
Danoflox msds (fiche de securite des materiaux)
Danoflox Synthese de reference
I. Hayakava et al., US. 4,382,892 (1983)
Danoflox Poids moleculaire
361.368 g/mol
Danoflox Point de fusion
250-257 oC
Danoflox H2O Solubilite
28,3 mg / ml
Danoflox Etat
Solid
Danoflox LogP
1.268
Danoflox Formes pharmaceutiques
Solution (injection IV); Tablet; gouttes ophtalmiques
Danoflox Indication
Pour le traitement des infections (voies respiratoires, les reins, la peau, des tissus mous, infections urinaires), l'urètre et le col de l'utérus gonorrhée.
Danoflox Pharmacologie
Ofloxacine est un antibiotique de la famille des quinolones / fluoroquinolones. Ofloxacine est bactéricide et son mode d'action repose sur le blocage de la réplication de l'ADN bactérien en se liant à une enzyme appelée ADN gyrase, qui permet la détorsion nécessaire pour répliquer une double hélice d'ADN en deux. Notamment le médicament a une affinité 100 fois plus élevée pour l'ADN gyrase bactérienne que pour les mammifères. Ofloxacine est un large spectre antibiotique actif contre les bactéries tant Gram-positives et gram-négatives.
Danoflox Absorption
La biodisponibilité de l'ofloxacine dans la formulation comprimé est d'environ 98%
Danoflox Toxicite
DL50 = 5450 mg / kg (par voie orale chez la souris)
Danoflox Information pour les patients
PATIENT INFORMATION
Patients should be advised:
- to drink fluids liberally;
- that mineral supplements, vitamins with iron or minerals, calcium- , aluminum-, or magnesium-based
antacids, sucralfate or Videx�, (Didanosine), chewable/buffered tablets or the pediatric powder for
oral solution should not be taken within the two-hour period before or within the two-hour period after
taking ofloxacin (See Drug Interactions);
- that ofloxacin can be taken without regard to meals;
- that ofloxacin may cause neurologic adverse effects (e. g. , dizziness, lightheadedness) and that
patients should know how they react to ofloxacin before they operate an automobile or machinery or
engage in activities requiring mental alertness and coordination
- to discontinue treatment and inform their physician if they experience pain, inflammation, or rupture
of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture
has been confidently excluded;
- that ofloxacin may be associated with hypersensitivity reactions, even following the first dose, to
discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat,
difficulty in swallowing or breathing, any swelling suggesting angioedema (e. g. , swelling of the lips,
tongue, face; tightness of the throat, hoarseness), or any other symptom of an allergic reaction
- to avoid excessive sunlight or artificial ultraviolet light while receiving ofloxacin and to discontinue
therapy if phototoxicity (e. g. , skin eruption) occurs;
- that if they are diabetic and are being treated with insulin or an oral hypoglycemic drug, to discontinue
ofloxacin immediately if a hypoglycemic reaction occurs and consult a physician
- that convulsions have been reported in patients taking quinolones, including ofloxacin, and to notify their
physician before taking this drug if there is a history of this condition.
Patients should be advised:
- to drink fluids liberally;
- that mineral supplements, vitamins with iron or minerals, calcium- , aluminum-, or magnesium-based
antacids, sucralfate or Videx�, (Didanosine), chewable/buffered tablets or the pediatric powder for
oral solution should not be taken within the two-hour period before or within the two-hour period after
taking ofloxacin (See Drug Interactions);
- that ofloxacin can be taken without regard to meals;
- that ofloxacin may cause neurologic adverse effects (e. g. , dizziness, lightheadedness) and that
patients should know how they react to ofloxacin before they operate an automobile or machinery or
engage in activities requiring mental alertness and coordination
- to discontinue treatment and inform their physician if they experience pain, inflammation, or rupture
of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture
has been confidently excluded;
- that ofloxacin may be associated with hypersensitivity reactions, even following the first dose, to
discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat,
difficulty in swallowing or breathing, any swelling suggesting angioedema (e. g. , swelling of the lips,
tongue, face; tightness of the throat, hoarseness), or any other symptom of an allergic reaction
- to avoid excessive sunlight or artificial ultraviolet light while receiving ofloxacin and to discontinue
therapy if phototoxicity (e. g. , skin eruption) occurs;
- that if they are diabetic and are being treated with insulin or an oral hypoglycemic drug, to discontinue
ofloxacin immediately if a hypoglycemic reaction occurs and consult a physician
- that convulsions have been reported in patients taking quinolones, including ofloxacin, and to notify their
physician before taking this drug if there is a history of this condition.
Danoflox Organismes affectes
Bactéries entériques et d'autres eubactéries