Venen (TN)




Venen (TN) Brand names, Venen (TN) Analogs
Venen (TN) Brand Names Mixture
- Actifed DM Syrup (dextromethorphan hydrobromide + pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Actifed DM Tablets (dextromethorphan hydrobromide + pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Actifed Plus Caplet (acetaminophen + pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Actifed Plus Caplets (acetaminophen + pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Actifed Syrup (pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Actifed Tablets (pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Coactifed (codeine phosphate + guaifenesin + pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Coactifed (codeine phosphate + pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Cotrifed (codeine phosphate + pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Covan Syrup (codeine phosphate + pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Nasal Decongestant Antihistamine (pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Ratio-Cotridin (codeine phosphate + pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Ratio-Cotridin Expectorant (codeine phosphate + guaifenesin + pseudoephedrine hydrochloride + triprolidine hydrochloride)
- Triprofed Tab (pseudoephedrine hydrochloride + triprolidine hydrochloride)
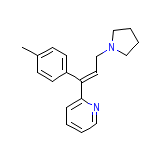
Venen (TN) Chemical_Formula
C19H22N2
Venen (TN) RX_link
No information avaliable
Venen (TN) fda sheet
Venen (TN) msds (material safety sheet)
Venen (TN) Synthesis Reference
No information avaliable
Venen (TN) Molecular Weight
278.391 g/mol
Venen (TN) Melting Point
60 oC
Venen (TN) H2O Solubility
74.9 mg/L
Venen (TN) State
Solid
Venen (TN) LogP
3.729
Venen (TN) Dosage Forms
Syrup; Tablet
Venen (TN) Indication
For the treatment of hay fever, urticaria (hives), and other allergic systems.
Venen (TN) Pharmacology
In allergic reactions an allergen interacts with and cross-links surface IgE antibodies on mast cells and basophils. Once the mast cell-antibody-antigen complex is formed, a complex series of events occurs that eventually leads to cell-degranulation and the release of histamine (and other chemical mediators) from the mast cell or basophil. Once released, histamine can react with local or widespread tissues through histamine receptors. Histamine, acting on H1-receptors, produces pruritis, vasodilatation, hypotension, flushing, headache, tachycardia, and bronchoconstriction. Histamine also increases vascular permeability and potentiates pain. Triprolidine, is a histamine H1 antagonist that competes with histamine for the normal H1-receptor sites on effector cells of the gastrointestinal tract, blood vessels and respiratory tract. It provides effective, temporary relief of sneezing, watery and itchy eyes, and runny nose due to hay fever and other upper respiratory allergies.
Venen (TN) Absorption
Rapidly absorbed in the intestinal tract.
Venen (TN) side effects and Toxicity
Symptoms of overdose include drowsiness, weakness, inco-ordination, difficulty with micturition, respiratory depression, hypotension, agitation, irritability, convulsions, hypertension, palpitation and tachycardia.
Venen (TN) Patient Information
Stop taking triprolidine 4 days before you have an allergy skin test.
Drink water frequently or use ice chips, sugarless candy, or sugarless gum if dry mouth occurs. Coffee or tea may
reduce the common side effect of drowsiness.
Venen (TN) Organisms Affected
Humans and other mammals