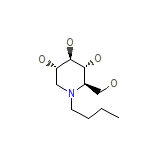
Miglustat




Miglustat Brand names, Miglustat Analogs
Miglustat Brand Names Mixture
- No information avaliable
Miglustat Chemical_Formula
C10H21NO4
Miglustat RX_link
http://www.rxlist.com/cgi/generic3/zavesca.htm
Miglustat fda sheet
Miglustat msds (material safety sheet)
Miglustat Synthesis Reference
No information avaliable
Miglustat Molecular Weight
219.278 g/mol
Miglustat Melting Point
169 - 172 oC
Miglustat H2O Solubility
Highly soluble in water (>1000 mg/mL as a free base).
Miglustat State
Solid
Miglustat LogP
-1.087
Miglustat Dosage Forms
Capsules (100-mg) for oral administration
Miglustat Indication
For the treatment of adult patients with mild to moderate type 1 Gaucher disease for whom enzyme replacement therapy is not a therapeutic option (e.g. due to constraints such as allergy, hypersensitivity, or poor venous access).
Miglustat Pharmacology
Miglustat is an N-alkylated imino sugar, a synthetic analogue of D-glucose. Miglustat is an inhibitor of the enzyme glucosylceramide synthase, which is a glucosyl transferase enzyme responsible for the first step in the synthesis of most glycosphingolipids.
Miglustat Absorption
Mean oral bioavailability is 97%.
Miglustat side effects and Toxicity
Miglustat has been administered at doses of up to 3000 mg/day (approximately 10 times the recommended starting dose administered to Gaucher patients) for up to six months in Human Immunodeficiency Virus (HIV)-positive patients. Adverse events observed in the HIV studies included granulocytopenia, dizziness, and paresthesia. Leukopenia and neutropenia have also been observed in a similar group of patients receiving 800 mg/day or above.
Miglustat Patient Information
Miglustat Organisms Affected
Humans and other mammals