Quinaglute Dura-Tabs




Categoria
Quinaglute Dura-Tabs Nombres de marca, Quinaglute Dura-Tabs Analogos
- Apo-Quinidine
- Biquin Durules
- Cardioquin
- Chinidin
- Cin-Quin
- Coccinine
- Conchinin
- Conchinine
- Conquinine
- Duraquin
- Kinidin
- Novoquinidin
- Pitayin
- Pitayine
- Quin-Release
- Quinact
- Quinaglute
- Quinaglute Dura-Tabs
- Quinalan
- Quinate
- Quinatime
- Quindine
- Quinicardine
- Quinidex
- Quinidex Extentabs
- Quinidine Gluconate
- Quinidine Sulfate
- Quinine, Polymers
- Quinora
Quinaglute Dura-Tabs Marca los nombres de mezcla
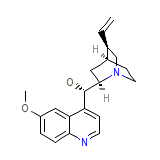
Quinaglute Dura-Tabs Formula quimica
C20H24N2O2
Quinaglute Dura-Tabs RX enlace
http://www.rxlist.com/cgi/generic/quinidine.htm
Quinaglute Dura-Tabs FDA hoja
Quinaglute Dura-Tabs MSDS (hoja de seguridad de materiales)
Quinaglute Dura-Tabs Sintesis de referencia
No hay información disponible
Quinaglute Dura-Tabs Peso molecular
324.417 g/mol
Quinaglute Dura-Tabs Punto de fusion
174 oC
Quinaglute Dura-Tabs H2O Solubilidad
140 mg / L
Quinaglute Dura-Tabs Estado
Solid
Quinaglute Dura-Tabs LogP
2.534
Quinaglute Dura-Tabs Formas de dosificacion
Solución
Quinaglute Dura-Tabs Indicacion
Para el tratamiento de pre-excitación ventricular y arritmias cardíacas
Quinaglute Dura-Tabs Farmacologia
Quinidina, un anticonvulsivo fenitoína, se usa solo o junto con fenobarbital u otros anticonvulsivos para gestionar crisis tónico-clónicas, ataques psicomotores, los síndromes de dolor neuropático diabético incluyendo neuropatía, inducidas por digitálicos arritmias cardíacas y arritmias cardíacas asociadas con prolongación del intervalo QT.
Quinaglute Dura-Tabs Absorcion
No hay información disponible
Quinaglute Dura-Tabs Toxicidad
No hay información disponible
Quinaglute Dura-Tabs Informacion de Pacientes
Before prescribing QUINAGLUTE® as prophylaxis against recurrence of atrial fibrillation, the physician should inform the patient of the risks and benefits to be expected. Discussion should include the facts
- that the goal of therapy will be a reduction (probably not to zero) in the frequency of episodes of atrial fibrillation; and
- that reduced frequency of fibrillatory episodes may be expected, if achieved, to bring symptomatic benefit; but
- that no data are available to show that reduced frequency of fibrillatory episodes will reduce the risks of irreversible harm through stroke or death; and in fact
- that such data as are available suggest that treatment with QUINAGLUTE® is likely to increase the patient's risk of death.
To confirm whether this is the most current prescribing information available on Quinaglute®, or to obtain the most current prescribing information, please call Berlex Laboratories at 1-888-BERLEX-4 (choose option #4, Product Usage Information).
Quinaglute Dura-Tabs Organismos afectados
Humanos y otros mamíferos