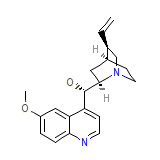
Quinaglute Dura-Tabs




Quinaglute Dura-Tabs Brand names, Quinaglute Dura-Tabs Analogs
- Apo-Quinidine
- Biquin Durules
- Cardioquin
- Chinidin
- Cin-Quin
- Coccinine
- Conchinin
- Conchinine
- Conquinine
- Duraquin
- Kinidin
- Novoquinidin
- Pitayin
- Pitayine
- Quin-Release
- Quinact
- Quinaglute
- Quinaglute Dura-Tabs
- Quinalan
- Quinate
- Quinatime
- Quindine
- Quinicardine
- Quinidex
- Quinidex Extentabs
- Quinidine Gluconate
- Quinidine Sulfate
- Quinine, Polymers
- Quinora
Quinaglute Dura-Tabs Brand Names Mixture
- No information avaliable
Quinaglute Dura-Tabs Chemical_Formula
C20H24N2O2
Quinaglute Dura-Tabs RX_link
http://www.rxlist.com/cgi/generic/quinidine.htm
Quinaglute Dura-Tabs fda sheet
Quinaglute Dura-Tabs msds (material safety sheet)
Quinaglute Dura-Tabs Synthesis Reference
No information avaliable
Quinaglute Dura-Tabs Molecular Weight
324.417 g/mol
Quinaglute Dura-Tabs Melting Point
174 oC
Quinaglute Dura-Tabs H2O Solubility
140 mg/L
Quinaglute Dura-Tabs State
Solid
Quinaglute Dura-Tabs LogP
2.534
Quinaglute Dura-Tabs Dosage Forms
Solution
Quinaglute Dura-Tabs Indication
For the treatment of ventricular pre-excitation and cardiac dysrhythmias
Quinaglute Dura-Tabs Pharmacology
Quinidine, a hydantoin anticonvulsant, is used alone or with phenobarbital or other anticonvulsants to manage tonic-clonic seizures, psychomotor seizures, neuropathic pain syndromes including diabetic neuropathy, digitalis-induced cardiac arrhythmias, and cardiac arrhythmias associated with QT-interval prolongation.
Quinaglute Dura-Tabs Absorption
No information avaliable
Quinaglute Dura-Tabs side effects and Toxicity
No information avaliable
Quinaglute Dura-Tabs Patient Information
Before prescribing QUINAGLUTE® as prophylaxis against recurrence of atrial fibrillation, the physician should inform the patient of the risks and benefits to be expected. Discussion should include the facts
- that the goal of therapy will be a reduction (probably not to zero) in the frequency of episodes of atrial fibrillation; and
- that reduced frequency of fibrillatory episodes may be expected, if achieved, to bring symptomatic benefit; but
- that no data are available to show that reduced frequency of fibrillatory episodes will reduce the risks of irreversible harm through stroke or death; and in fact
- that such data as are available suggest that treatment with QUINAGLUTE® is likely to increase the patient's risk of death.
To confirm whether this is the most current prescribing information available on Quinaglute®, or to obtain the most current prescribing information, please call Berlex Laboratories at 1-888-BERLEX-4 (choose option #4, Product Usage Information).
Quinaglute Dura-Tabs Organisms Affected
Humans and other mammals