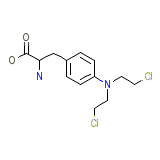
Sarcolysine




Sarcolysine Brand names, Sarcolysine Analogs
Sarcolysine Brand Names Mixture
- No information avaliable
Sarcolysine Chemical_Formula
C13H18Cl2N2O2
Sarcolysine RX_link
http://www.rxlist.com/cgi/generic2/melph.htm
Sarcolysine fda sheet
Sarcolysine msds (material safety sheet)
Sarcolysine Synthesis Reference
Sen, A.K., et al.; Indian J.Chem.Sect.B; 22; 9; 939-941(1983)
Sarcolysine Molecular Weight
305.2 g/mol
Sarcolysine Melting Point
182.5 oC
Sarcolysine H2O Solubility
< 0.1 g/100 mL at 22°C
Sarcolysine State
Solid
Sarcolysine LogP
-0.432
Sarcolysine Dosage Forms
Tablet; Solution (for injection)
Sarcolysine Indication
For the palliative treatment of multiple myeloma and for the palliation of non-resectable epithelial carcinoma of the ovary.
Sarcolysine Pharmacology
Melphalan is an antineoplastic in the class of alkylating agents and is used to treat various forms of cancer. Alkylating agents are so named because of their ability to add alkyl groups to many electronegative groups under conditions present in cells. They stop tumor growth by cross-linking guanine bases in DNA double-helix strands - directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. In addition, these drugs add methyl or other alkyl groups onto molecules where they do not belong which in turn inhibits their correct utilization by base pairing and causes a miscoding of DNA. Alkylating agents are cell cycle-nonspecific. Alkylating agents work by three different mechanisms all of which achieve the same end result - disruption of DNA function and cell death.
Sarcolysine Absorption
Incomplete, variable, 25-89% post oral dose
Sarcolysine side effects and Toxicity
Vomiting, ulceration of the mouth, diarrhea, and hemorrhage of the gastrointestinal tract; The principal toxic effect is bone marrow suppression. LD50=11.2 mg/kg (orally in rat)
Sarcolysine Patient Information
PATIENT INFORMATION
Patients should be informed that the major toxicities of ALKERAN are related
to bone marrow suppression, hypersensitivity reactions, gastrointestinal
toxicity, and pulmonary toxicity. The major long-term toxicities are related
to infertility and secondary malignancies. Patients should never be allowed
to take the drug without close medical supervision and should be advised to
consult their physician if they experience skin rash, vasculitis, bleeding,
fever, persistent cough, nausea, vomiting, amenorrhea, weight loss, or unusual
lumps/masses. Women of childbearing potential should be advised to avoid
becoming pregnant.
Laboratory Tests: Periodic complete blood counts with differentials should be
performed during the course of treatment with ALKERAN. At least one determination
should be obtained prior to each treatment course. Patients should be observed
closely for consequences of bone marrow suppression, which include severe infections,
bleeding, and symptomatic anemia.
Patients should be informed that the major toxicities of ALKERAN are related
to bone marrow suppression, hypersensitivity reactions, gastrointestinal
toxicity, and pulmonary toxicity. The major long-term toxicities are related
to infertility and secondary malignancies. Patients should never be allowed
to take the drug without close medical supervision and should be advised to
consult their physician if they experience skin rash, vasculitis, bleeding,
fever, persistent cough, nausea, vomiting, amenorrhea, weight loss, or unusual
lumps/masses. Women of childbearing potential should be advised to avoid
becoming pregnant.
Laboratory Tests: Periodic complete blood counts with differentials should be
performed during the course of treatment with ALKERAN. At least one determination
should be obtained prior to each treatment course. Patients should be observed
closely for consequences of bone marrow suppression, which include severe infections,
bleeding, and symptomatic anemia.
Sarcolysine Organisms Affected
Humans and other mammals