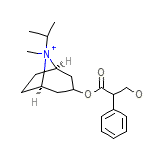
Rinoberen




Rinoberen Brand names, Rinoberen Analogs
Rinoberen Brand Names Mixture
- Combivent Inhalation Aerosol (Ipratropium Bromide + Salbutamol Sulfate)
- Combivent Inhalation Solution (Ipratropium Bromide + Salbutamol Sulfate)
- Duovent Udv (Fenoterol Hydrobromide + Ipratropium Bromide)
- Ratio-Ipra Sal Udv (Ipratropium Bromide + Salbutamol Sulfate)
Rinoberen Chemical_Formula
C20H30NO3
Rinoberen RX_link
http://www.rxlist.com/cgi/generic/ipratrop.htm
Rinoberen fda sheet
Rinoberen msds (material safety sheet)
Rinoberen Synthesis Reference
K. Zeile et al.,U.S. Pat. 3,505,337 (1970)
Rinoberen Molecular Weight
332.457 g/mol
Rinoberen Melting Point
230-232oC
Rinoberen H2O Solubility
Freely soluble
Rinoberen State
Solid
Rinoberen LogP
No information avaliable
Rinoberen Dosage Forms
Aerosol (with propellants); Liquid; Metered-dose (aerosol); Metered-dose (pump); Solution
Rinoberen Indication
For maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema.
Rinoberen Pharmacology
Ipratropium bromide, a synthetic ammonium compound structurally similar to atropine, is used as a bronchodilator in the management of cholinergic-mediated bronchospasm associated with chronic obstructive pulmonary disease and in the treatment of rhinorrhea associated with the common cold or with allergic or nonallergic seasonal rhinitis.
Rinoberen Absorption
Inhalation (local)-minimal; Nasal-rapid and minimal
Rinoberen side effects and Toxicity
LD50=1001mg/kg (orally in mice)
Rinoberen Patient Information
Rinoberen Organisms Affected
Humans and other mammals