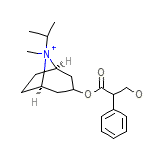
Atrovent Nasal




Atrovent Nasal Brand names, Atrovent Nasal Analogs
Atrovent Nasal Brand Names Mixture
- Combivent Inhalation Aerosol (Ipratropium Bromide + Salbutamol Sulfate)
- Combivent Inhalation Solution (Ipratropium Bromide + Salbutamol Sulfate)
- Duovent Udv (Fenoterol Hydrobromide + Ipratropium Bromide)
- Ratio-Ipra Sal Udv (Ipratropium Bromide + Salbutamol Sulfate)
Atrovent Nasal Chemical_Formula
C20H30NO3
Atrovent Nasal RX_link
http://www.rxlist.com/cgi/generic/ipratrop.htm
Atrovent Nasal fda sheet
Atrovent Nasal msds (material safety sheet)
Atrovent Nasal Synthesis Reference
K. Zeile et al.,U.S. Pat. 3,505,337 (1970)
Atrovent Nasal Molecular Weight
332.457 g/mol
Atrovent Nasal Melting Point
230-232oC
Atrovent Nasal H2O Solubility
Freely soluble
Atrovent Nasal State
Solid
Atrovent Nasal LogP
No information avaliable
Atrovent Nasal Dosage Forms
Aerosol (with propellants); Liquid; Metered-dose (aerosol); Metered-dose (pump); Solution
Atrovent Nasal Indication
For maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema.
Atrovent Nasal Pharmacology
Ipratropium bromide, a synthetic ammonium compound structurally similar to atropine, is used as a bronchodilator in the management of cholinergic-mediated bronchospasm associated with chronic obstructive pulmonary disease and in the treatment of rhinorrhea associated with the common cold or with allergic or nonallergic seasonal rhinitis.
Atrovent Nasal Absorption
Inhalation (local)-minimal; Nasal-rapid and minimal
Atrovent Nasal side effects and Toxicity
LD50=1001mg/kg (orally in mice)
Atrovent Nasal Patient Information
Atrovent Nasal Organisms Affected
Humans and other mammals