L-5103 Lepetit




L-5103 Lepetit Brand names, L-5103 Lepetit Analogs
L-5103 Lepetit Brand Names Mixture
- Rifater (Isoniazid + Pyrazinaamide + Rifampin)
- Rifamate (Rifampin + Isoniazid)
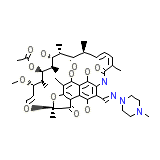
L-5103 Lepetit Chemical_Formula
L-5103 Lepetit RX_link
L-5103 Lepetit fda sheet
L-5103 Lepetit msds (material safety sheet)
L-5103 Lepetit Synthesis Reference
L-5103 Lepetit Molecular Weight
L-5103 Lepetit Melting Point
L-5103 Lepetit H2O Solubility
L-5103 Lepetit State
L-5103 Lepetit LogP
L-5103 Lepetit Dosage Forms
L-5103 Lepetit Indication
L-5103 Lepetit Pharmacology
L-5103 Lepetit Absorption
L-5103 Lepetit side effects and Toxicity
L-5103 Lepetit Patient Information
The patient should be told that rifampin may produce a reddish coloration of the urine, sweat, sputum, and tears, and the patient should be forewarned of this. Soft contact lenses may be permanently stained. The patients should be advised that the reliability of oral or other systemic hormonal contraceptives may be affected; consideration should be given to using alternative contraceptive measures. Patients should be instructed to take rifampin either 1 hour before or 2 hours after a meal with a full glass of water. Patients should be instructed to notify their physicians promptly if they experience any of the following: fever, loss of appetite, malaise, nausea and vomiting, darkened urine, yellowish discoloration of the skin and eyes, and pain or swelling of the joints. Compliance with the full course of therapy must be emphasized, and the importance of not missing any doses must be stressed.