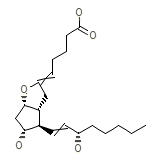
Edrophonium Chloride




Edrophonium Chloride Brand names, Edrophonium Chloride Analogs
Edrophonium Chloride Brand Names Mixture
- No information avaliable
Edrophonium Chloride Chemical_Formula
C20H32O5
Edrophonium Chloride RX_link
http://www.rxlist.com/cgi/generic/flolan.htm
Edrophonium Chloride fda sheet
Edrophonium Chloride msds (material safety sheet)
Edrophonium Chloride Synthesis Reference
No information avaliable
Edrophonium Chloride Molecular Weight
352.465 g/mol
Edrophonium Chloride Melting Point
No information avaliable
Edrophonium Chloride H2O Solubility
No information avaliable
Edrophonium Chloride State
Solid
Edrophonium Chloride LogP
2.543
Edrophonium Chloride Dosage Forms
Powder for solution
Edrophonium Chloride Indication
For the long-term intravenous treatment of primary pulmonary hypertension and pulmonary hypertension associated with the scleroderma spectrum of disease in NYHA Class III and Class IV patients who do not respond adequately to conventional therapy.
Edrophonium Chloride Pharmacology
Epoprostenol has two major pharmacological actions: (1) direct vasodilation of pulmonary and systemic arterial vascular beds, and (2) inhibition of platelet aggregation. In animals, the vasodilatory effects reduce right and left ventricular afterload and increase cardiac output and stroke volume. The effect of epoprostenol on heart rate in animals varies with dose. At low doses, there is vagally mediated brudycardia, but at higher doses, epoprostenol causes reflex tachycardia in response to direct vasodilation and hypotension. No major effects on cardiac conduction have been observed. Additional pharmacologic effects of epoprostenol in animals include bronchodilation, inhibition of gastric acid secretion, and decreased gastric emptying. No available chemical assay is sufficiently sensitive and specific to assess the in vivo human pharmacokinetics of epoprostenol.
Edrophonium Chloride Absorption
No information avaliable
Edrophonium Chloride side effects and Toxicity
Symptoms of overdose are extensions of its dose-limiting pharmacologic effects and include flushing, headache, hypotension, nausea, vomiting, and diarrhea. Most events were self-limiting and resolved with reduction or withholding of epoprostenol. Single intravenous doses at 10 and 50 mg/kg (2703 and 27,027 times the recommended acute phase human dose based on body surface area) were lethal to mice and rats, respectively. Symptoms of acute toxicity were hypoactivity, ataxia, loss of righting reflex, deep slow breathing, and hypothermia.
Edrophonium Chloride Patient Information
Edrophonium Chloride Organisms Affected
Humans and other mammals