Singular




Categorie
Singular Les marques, Singular Analogs
Singular Les marques melange
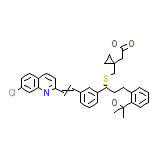
Singular Formule chimique
C35H36ClNO3S
Singular RX lien
http://www.rxlist.com/cgi/generic3/monteluk.htm
Singular FDA fiche
Singular msds (fiche de securite des materiaux)
Singular Synthese de reference
ML Belley et al. Eur. Pat. Appl. 480,717 (1992)
Singular Poids moleculaire
586.184 g/mol
Singular Point de fusion
No information avaliable
Singular H2O Solubilite
Aucune information disponible
Singular Etat
Solid
Singular LogP
8.488
Singular Formes pharmaceutiques
Tablet (oral)
Singular Indication
Pour le traitement de l'asthme
Singular Pharmacologie
Le montélukast, zafirlukast, comme, est un antagoniste des récepteurs des leucotriènes utilisé comme une alternative aux médicaments anti-inflammatoires dans la gestion et le traitement de l'asthme et l'exercice bronchospasme induit par (BEI). Contrairement zafirlukast, montélukast n'a pas inhibé le CYP2C9 ou le CYP3A4 et, par conséquent, ne devrait pas affecter la clairance hépatique des médicaments métabolisés par ces enzymes.
Singular Absorption
Rapidement absorbé après administration orale (biodisponibilité est de 64%)
Singular Toxicite
Les effets secondaires comprennent des céphalées, des douleurs abdominales ou de l'estomac, la toux, les douleurs dentaires, des vertiges, fièvre, brûlures d'estomac, éruption cutanée, nez bouché, faiblesse ou fatigue inhabituelle.
Singular Information pour les patients
General
- Patients should be advised to take montelukast daily as prescribed, even when they are asymptomatic, as well as during periods of worsening asthma, and to contact their physicians if their asthma is not well controlled.
- Patients should be advised that oral tablets of montelukast are not for the treatment of acute asthma attacks. They should have appropriate short-acting inhaled b-agonist medication available to treat asthma exacerbations.
- Patients should be advised that, while using montelukast, medical attention should be sought if short-acting inhaled bronchodilators are needed more often than usual, or if more than the maximum number of inhalations of short-acting bronchodilator treatment prescribed for 24-hour period are needed.
- Patients receiving montelukast should be instructed not to decrease the dose or stop taking any other antiasthma medications unless instructed by a physician.
- Patients who have exacerbations of asthma after exercise should be instructed to continue to use their usual regimen of inhaled b-agonists as prophylaxis unless otherwise instructed by their physician. All patients should have available for rescue a short-acting inhaled b-agonist.
- Patients with known aspirin sensitivity should be advised to continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast.
Phenylketonurics: Phenylketonuric patients should be informed that the chewable tablet contains phenylalanine (a component of aspartame) 0.842 mg per 5-mg chewable tablet.
Singular Organismes affectes
Les humains et autres mammifères