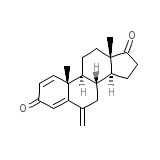
Exemestance




Categorie
Exemestance Les marques, Exemestance Analogs
Exemestance Les marques melange
Exemestance Formule chimique
C20H24O2
Exemestance RX lien
http://www.rxlist.com/cgi/generic3/exemest.htm
Exemestance FDA fiche
Exemestance msds (fiche de securite des materiaux)
Exemestance Synthese de reference
Pfizer Canada Inc, numéro de brevet: 1277656
Exemestance Poids moleculaire
296.403 g/mol
Exemestance Point de fusion
155.13oC
Exemestance H2O Solubilite
Non-solubles
Exemestance Etat
Solid
Exemestance LogP
4.222
Exemestance Formes pharmaceutiques
Tablet
Exemestance Indication
Pour le traitement du cancer du sein avancé chez les femmes ménopausées dont la maladie a progressé après un traitement au tamoxifène.
Exemestance Pharmacologie
L'aromatase est une enzyme qui convertit les hormones d'oestrogène dans les glandes surrénales du corps. Les inhibiteurs de l'aromatase (IA) sont des médicaments qui réduisent les niveaux d'oestrogène en bloquant l'action de l'aromatase dans la surrénale glandes. L'AIS sélective (ISC) de réduire sélectivement des niveaux d'oestrogène sans interférer avec les niveaux des autres hormones stéroïdes qui sont produites par la glande surrénale. Médicaments de cette classe comprennent l'anastrozole (Arimidex ™), le létrozole (Femara ™) et l'exémestane (Aromasin ™).
Exemestance Absorption
42%
Exemestance Toxicite
Convulsions
Exemestance Information pour les patients
PATIENT INFORMATION
If clinically advisable, patients receiving OxyContin� (oxycodone hydrochloride controlled-release) tablets or
their caregivers should be given the following information by the physician, nurse, pharmacist, or caregiver:
1. Patients should be aware that OxyContin� tablets contain oxycodone, which is a morphine-like substance.
2. Patients should be advised that OxyContin� tablets were designed to work properly only if swallowed whole.
OxyContin� tablets will release all their contents at once if broken, chewed, or crushed, resulting in a risk of
fatal overdose.
3. Patients should be advised to report episodes of breakthrough pain and adverse experiences occurring during
therapy. Individualization of dosage is essential to make optimal use of this medication.
4. Patients should be advised not to adjust the dose of OxyContin� without consulting the prescribing professional.
5. Patients should be advised that OxyContin� may impair mental and/or physical ability required for the performance
of potentially hazardous tasks (e.g., driving, operating heavy machinery).
6. Patients should not combine OxyContin� with alcohol or other central nervous system depressants (sleep aids,
tranquilizers) except by the orders of the prescribing physician, because dangerous additive effects may occur,
resulting in serious injury or death.
7. Women of childbearing potential who become, or are planning to become, pregnant should be advised to consult their
physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
8. Patients should be advised that OxyContin� is a potential drug of abuse. They should protect it from theft, and it
should never be given to anyone other than the individual for whom it was prescribed.
9. Patients should be advised that they may pass empty matrix "ghosts" (tablets) via colostomy or in the stool, and that
this is of no concern since the active medication has already been absorbed.
10. Patients should be advised that if they have been receiving treatment with OxyContin� for more than a few weeks and
cessation of therapy is indicated, it may be appropriate to taper the OxyContin� dose, rather than abruptly discontinue
it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a
gradual discontinuation of the medication.
11. Patients should be instructed to keep OxyContin� in a secure place out of the reach of children. When OxyContin� is
no longer needed, the unused tablets should be destroyed by flushing down the toilet.
If clinically advisable, patients receiving OxyContin� (oxycodone hydrochloride controlled-release) tablets or
their caregivers should be given the following information by the physician, nurse, pharmacist, or caregiver:
1. Patients should be aware that OxyContin� tablets contain oxycodone, which is a morphine-like substance.
2. Patients should be advised that OxyContin� tablets were designed to work properly only if swallowed whole.
OxyContin� tablets will release all their contents at once if broken, chewed, or crushed, resulting in a risk of
fatal overdose.
3. Patients should be advised to report episodes of breakthrough pain and adverse experiences occurring during
therapy. Individualization of dosage is essential to make optimal use of this medication.
4. Patients should be advised not to adjust the dose of OxyContin� without consulting the prescribing professional.
5. Patients should be advised that OxyContin� may impair mental and/or physical ability required for the performance
of potentially hazardous tasks (e.g., driving, operating heavy machinery).
6. Patients should not combine OxyContin� with alcohol or other central nervous system depressants (sleep aids,
tranquilizers) except by the orders of the prescribing physician, because dangerous additive effects may occur,
resulting in serious injury or death.
7. Women of childbearing potential who become, or are planning to become, pregnant should be advised to consult their
physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
8. Patients should be advised that OxyContin� is a potential drug of abuse. They should protect it from theft, and it
should never be given to anyone other than the individual for whom it was prescribed.
9. Patients should be advised that they may pass empty matrix "ghosts" (tablets) via colostomy or in the stool, and that
this is of no concern since the active medication has already been absorbed.
10. Patients should be advised that if they have been receiving treatment with OxyContin� for more than a few weeks and
cessation of therapy is indicated, it may be appropriate to taper the OxyContin� dose, rather than abruptly discontinue
it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a
gradual discontinuation of the medication.
11. Patients should be instructed to keep OxyContin� in a secure place out of the reach of children. When OxyContin� is
no longer needed, the unused tablets should be destroyed by flushing down the toilet.
Exemestance Organismes affectes
Les humains et autres mammifères