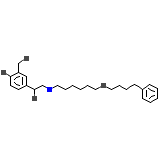
Astmerole




Categoria
Astmerole Nombres de marca, Astmerole Analogos
Astmerole Marca los nombres de mezcla
Astmerole Formula quimica
C25H37NO4
Astmerole RX enlace
http://www.rxlist.com/cgi/generic/salmeterol.htm
Astmerole FDA hoja
Astmerole MSDS (hoja de seguridad de materiales)
Astmerole Sintesis de referencia
SI Skidmore et al., Patente de EE.UU.. 4.992.474 (1991).
Astmerole Peso molecular
415.566 g/mol
Astmerole Punto de fusion
75.5-76.5 oC
Astmerole H2O Solubilidad
Muy poco soluble
Astmerole Estado
Solid
Astmerole LogP
5.022
Astmerole Formas de dosificacion
Inhaladores de dosis medidas (aerosol), en polvo
Astmerole Indicacion
Para el tratamiento del asma y enfermedad pulmonar obstructiva crónica (EPOC).
Astmerole Farmacologia
Salmeterol es una acción prolongada beta2-adrenérgico (LABA), por lo general sólo se prescribe para el asma persistente grave tras un tratamiento previo con un agonista beta de acción corta como el salbutamol y se prescribe al mismo tiempo con un corticosteroide, como la beclometasona. La principal diferencia notable de salmeterol con salbutamol es que la duración de la acción dura aproximadamente 12 horas en comparación con 4-6 horas de salbutamol. Cuando se usa regularmente todos los días como presecribed, salmeterol inhalados reduce el número y la severidad de los ataques de asma. Sin embargo, no es para su uso para aliviar un ataque de asma que ya ha comenzado. Salmeterol inhalado funciona como otros beta-2 agonistas, provocando broncodilatación mediante la relajación del músculo liso en las vías respiratorias con el fin de tratar la exacerbación del asma. Salmeterol es una acción similar a formoterol, formoterol sin embargo se ha demostrado que tiene un rápido comienzo de acción de salmeterol como resultado de una menor lipofilicidad, y también ha demostrado ser más potente - una dosis de formoterol 12 mg se ha demostrado que es equivalente a una dosis de 50 mg de salmeterol.
Astmerole Absorcion
Debido a la dosis terapéutica pequeños, los niveles sistémicos de salmeterol son muy bajos o indetectables después de la inhalación de dosis recomendadas.
Astmerole Toxicidad
Los síntomas de sobredosis incluyen angina (dolor de pecho), mareo, boca seca, fatiga, síntomas parecidos a la gripe, dolor de cabeza, irregularidades cardiacas, presión arterial alta o baja, azúcar en la sangre, insomnio, calambres musculares, náuseas, nerviosismo, taquicardia, convulsiones y temblores. Por vía oral, no se produjeron muertes en ratas a 1.000 mg / kg (aproximadamente 81.000 veces la dosis máxima recomendada por inhalación al día en adultos y aproximadamente 38.000 veces la dosis máxima recomendada inhalación diaria de los niños sobre una base mg/m2).
Astmerole Informacion de Pacientes
PATIENT INFORMATION
Patients being treated with SEREVENT DISKUS should receive the following information and instructions. This information is
intended to aid them in the safe and effective use of this medication. It is not a disclosure of all possible adverse or
intended effects.
It is important that patients understand how to use the DISKUS appropriately and how to use SEREVENT DISKUS in relation to
other asthma or COPD medications they are taking. Patients should be given the following information:
1. The action of SEREVENT DISKUS may last up to 12 hours or longer. The recommended dosage (1 inhalation twice daily, morning
and evening) should not be exceeded.
2. Most patients are able to taste or feel a dose delivered from SEREVENT DISKUS. However, whether or not patients are able to
sense delivery of a dose, you should instruct them not to exceed the recommended dose of 1 inhalation twice daily, morning and
evening. You should instruct them to contact you or the pharmacist if they have questions.
3. SEREVENT DISKUS is not meant to relieve acute asthma or COPD symptoms and extra doses should not be used for that purpose.
Acute symptoms should be treated with an inhaled, short-acting bronchodilator (the physician should provide the patient with
such medication and instruct the patient in how it should be used).
4. Patients should not stop therapy with SEREVENT DISKUS for asthma or COPD without physician/provider guidance since symptoms
may worsen after discontinuation.
5. �When used for the treatment of EIB, 1 inhalation of SEREVENT DISKUS should be taken 30 minutes before exercise.
� Additional doses of SEREVENT should not be used for 12 hours.
� Patients who are receiving SEREVENT DISKUS twice daily should not use additional SEREVENT for prevention of EIB.
6. The physician should be notified immediately if any of the following situations occur, which may be a sign of seriously
worsening asthma or COPD:
� Decreasing effectiveness of inhaled, short-acting beta2-agonists
� Need for more inhalations than usual of inhaled, short-acting beta2-agonists
� Significant decrease in PEF or lung function as outlined by the physician
� Use of 4 or more inhalations per day of a short-acting beta2-agonist for 2 or more days consecutively
� Use of more than 1 canister (200 inhalations per canister) of an inhaled, short-acting beta2-agonist in an 8-week period.
7. SEREVENT DISKUS should not be used as a substitute for oral or inhaled corticosteroids. The dosage of these medications should
not be changed and they should not be stopped without consulting the physician, even if the patient feels better after initiating
treatment with SEREVENT DISKUS.
8. Patients should be cautioned regarding adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid
heart rate, tremor, or nervousness.
9. When patients are prescribed SEREVENT DISKUS, other medications for asthma and COPD should be used only as directed by the
physician.
10. SEREVENT DISKUS should not be used with a spacer device.
11. Patients who are pregnant or nursing should contact the physician about the use of SEREVENT DISKUS.
12. Effective and safe use of SEREVENT DISKUS includes an understanding of the way that it should be used:
� Never exhale into the DISKUS.
� Never attempt to take the DISKUS apart.
� Always activate and use the DISKUS in a level, horizontal position.
� Never wash the mouthpiece or any part of the DISKUS. KEEP IT DRY.
� Always keep the DISKUS in a dry place.
� Discard 6 weeks after removal from the moisture-protective foil overwrap pouch or after all blisters have been used (when the
dose indicator reads "0" ), whichever comes first.
13. For the proper use of SEREVENT DISKUS and to attain maximum benefit, the patient should read and follow carefully the Patient's
Instructions for Use accompanying the product.
Patients being treated with SEREVENT DISKUS should receive the following information and instructions. This information is
intended to aid them in the safe and effective use of this medication. It is not a disclosure of all possible adverse or
intended effects.
It is important that patients understand how to use the DISKUS appropriately and how to use SEREVENT DISKUS in relation to
other asthma or COPD medications they are taking. Patients should be given the following information:
1. The action of SEREVENT DISKUS may last up to 12 hours or longer. The recommended dosage (1 inhalation twice daily, morning
and evening) should not be exceeded.
2. Most patients are able to taste or feel a dose delivered from SEREVENT DISKUS. However, whether or not patients are able to
sense delivery of a dose, you should instruct them not to exceed the recommended dose of 1 inhalation twice daily, morning and
evening. You should instruct them to contact you or the pharmacist if they have questions.
3. SEREVENT DISKUS is not meant to relieve acute asthma or COPD symptoms and extra doses should not be used for that purpose.
Acute symptoms should be treated with an inhaled, short-acting bronchodilator (the physician should provide the patient with
such medication and instruct the patient in how it should be used).
4. Patients should not stop therapy with SEREVENT DISKUS for asthma or COPD without physician/provider guidance since symptoms
may worsen after discontinuation.
5. �When used for the treatment of EIB, 1 inhalation of SEREVENT DISKUS should be taken 30 minutes before exercise.
� Additional doses of SEREVENT should not be used for 12 hours.
� Patients who are receiving SEREVENT DISKUS twice daily should not use additional SEREVENT for prevention of EIB.
6. The physician should be notified immediately if any of the following situations occur, which may be a sign of seriously
worsening asthma or COPD:
� Decreasing effectiveness of inhaled, short-acting beta2-agonists
� Need for more inhalations than usual of inhaled, short-acting beta2-agonists
� Significant decrease in PEF or lung function as outlined by the physician
� Use of 4 or more inhalations per day of a short-acting beta2-agonist for 2 or more days consecutively
� Use of more than 1 canister (200 inhalations per canister) of an inhaled, short-acting beta2-agonist in an 8-week period.
7. SEREVENT DISKUS should not be used as a substitute for oral or inhaled corticosteroids. The dosage of these medications should
not be changed and they should not be stopped without consulting the physician, even if the patient feels better after initiating
treatment with SEREVENT DISKUS.
8. Patients should be cautioned regarding adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid
heart rate, tremor, or nervousness.
9. When patients are prescribed SEREVENT DISKUS, other medications for asthma and COPD should be used only as directed by the
physician.
10. SEREVENT DISKUS should not be used with a spacer device.
11. Patients who are pregnant or nursing should contact the physician about the use of SEREVENT DISKUS.
12. Effective and safe use of SEREVENT DISKUS includes an understanding of the way that it should be used:
� Never exhale into the DISKUS.
� Never attempt to take the DISKUS apart.
� Always activate and use the DISKUS in a level, horizontal position.
� Never wash the mouthpiece or any part of the DISKUS. KEEP IT DRY.
� Always keep the DISKUS in a dry place.
� Discard 6 weeks after removal from the moisture-protective foil overwrap pouch or after all blisters have been used (when the
dose indicator reads "0" ), whichever comes first.
13. For the proper use of SEREVENT DISKUS and to attain maximum benefit, the patient should read and follow carefully the Patient's
Instructions for Use accompanying the product.
Astmerole Organismos afectados
Humanos y otros mamíferos