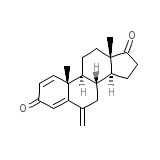
Aromasin




Categoria
Aromasin Nombres de marca, Aromasin Analogos
Aromasin Marca los nombres de mezcla
Aromasin Formula quimica
C20H24O2
Aromasin RX enlace
http://www.rxlist.com/cgi/generic3/exemest.htm
Aromasin FDA hoja
Aromasin MSDS (hoja de seguridad de materiales)
Aromasin Sintesis de referencia
Pfizer Canada Inc, el número de patente: 1277656
Aromasin Peso molecular
296.403 g/mol
Aromasin Punto de fusion
155.13oC
Aromasin H2O Solubilidad
No soluble
Aromasin Estado
Solid
Aromasin LogP
4.222
Aromasin Formas de dosificacion
Tableta
Aromasin Indicacion
Para el tratamiento del cáncer de mama avanzado en mujeres postmenopáusicas cuya enfermedad ha progresado después de tratamiento con tamoxifeno.
Aromasin Farmacologia
La aromatasa es una enzima que convierte las hormonas de estrógeno en las glándulas suprarrenales del cuerpo. Los inhibidores de la aromatasa (IA) son medicamentos que reducen los niveles de estrógeno mediante el bloqueo de la acción de la aromatasa en las glándulas suprarrenales las glándulas. Los inhibidores de la aromatasa selectivos (EFS) de forma selectiva a reducir los niveles de estrógeno sin interferir con los niveles de otras hormonas esteroides que son producidas por la glándula suprarrenal. Los medicamentos de esta clase son el anastrozol (Arimidex ™), el letrozol (Femara ™) y exemestano (Aromasin ™).
Aromasin Absorcion
42%
Aromasin Toxicidad
Convulsiones
Aromasin Informacion de Pacientes
PATIENT INFORMATION
If clinically advisable, patients receiving OxyContin� (oxycodone hydrochloride controlled-release) tablets or
their caregivers should be given the following information by the physician, nurse, pharmacist, or caregiver:
1. Patients should be aware that OxyContin� tablets contain oxycodone, which is a morphine-like substance.
2. Patients should be advised that OxyContin� tablets were designed to work properly only if swallowed whole.
OxyContin� tablets will release all their contents at once if broken, chewed, or crushed, resulting in a risk of
fatal overdose.
3. Patients should be advised to report episodes of breakthrough pain and adverse experiences occurring during
therapy. Individualization of dosage is essential to make optimal use of this medication.
4. Patients should be advised not to adjust the dose of OxyContin� without consulting the prescribing professional.
5. Patients should be advised that OxyContin� may impair mental and/or physical ability required for the performance
of potentially hazardous tasks (e.g., driving, operating heavy machinery).
6. Patients should not combine OxyContin� with alcohol or other central nervous system depressants (sleep aids,
tranquilizers) except by the orders of the prescribing physician, because dangerous additive effects may occur,
resulting in serious injury or death.
7. Women of childbearing potential who become, or are planning to become, pregnant should be advised to consult their
physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
8. Patients should be advised that OxyContin� is a potential drug of abuse. They should protect it from theft, and it
should never be given to anyone other than the individual for whom it was prescribed.
9. Patients should be advised that they may pass empty matrix "ghosts" (tablets) via colostomy or in the stool, and that
this is of no concern since the active medication has already been absorbed.
10. Patients should be advised that if they have been receiving treatment with OxyContin� for more than a few weeks and
cessation of therapy is indicated, it may be appropriate to taper the OxyContin� dose, rather than abruptly discontinue
it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a
gradual discontinuation of the medication.
11. Patients should be instructed to keep OxyContin� in a secure place out of the reach of children. When OxyContin� is
no longer needed, the unused tablets should be destroyed by flushing down the toilet.
If clinically advisable, patients receiving OxyContin� (oxycodone hydrochloride controlled-release) tablets or
their caregivers should be given the following information by the physician, nurse, pharmacist, or caregiver:
1. Patients should be aware that OxyContin� tablets contain oxycodone, which is a morphine-like substance.
2. Patients should be advised that OxyContin� tablets were designed to work properly only if swallowed whole.
OxyContin� tablets will release all their contents at once if broken, chewed, or crushed, resulting in a risk of
fatal overdose.
3. Patients should be advised to report episodes of breakthrough pain and adverse experiences occurring during
therapy. Individualization of dosage is essential to make optimal use of this medication.
4. Patients should be advised not to adjust the dose of OxyContin� without consulting the prescribing professional.
5. Patients should be advised that OxyContin� may impair mental and/or physical ability required for the performance
of potentially hazardous tasks (e.g., driving, operating heavy machinery).
6. Patients should not combine OxyContin� with alcohol or other central nervous system depressants (sleep aids,
tranquilizers) except by the orders of the prescribing physician, because dangerous additive effects may occur,
resulting in serious injury or death.
7. Women of childbearing potential who become, or are planning to become, pregnant should be advised to consult their
physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
8. Patients should be advised that OxyContin� is a potential drug of abuse. They should protect it from theft, and it
should never be given to anyone other than the individual for whom it was prescribed.
9. Patients should be advised that they may pass empty matrix "ghosts" (tablets) via colostomy or in the stool, and that
this is of no concern since the active medication has already been absorbed.
10. Patients should be advised that if they have been receiving treatment with OxyContin� for more than a few weeks and
cessation of therapy is indicated, it may be appropriate to taper the OxyContin� dose, rather than abruptly discontinue
it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a
gradual discontinuation of the medication.
11. Patients should be instructed to keep OxyContin� in a secure place out of the reach of children. When OxyContin� is
no longer needed, the unused tablets should be destroyed by flushing down the toilet.
Aromasin Organismos afectados
Humanos y otros mamíferos