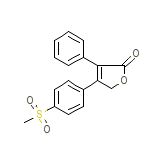
Vioxx




Vioxx Brand names, Vioxx Analogs
Vioxx Brand Names Mixture
- No information avaliable
Vioxx Chemical_Formula
C17H14O4S
Vioxx RX_link
http://www.rxlist.com/cgi/generic/rofecox.htm
Vioxx fda sheet
Vioxx msds (material safety sheet)
Vioxx Synthesis Reference
No information avaliable
Vioxx Molecular Weight
314.357 g/mol
Vioxx Melting Point
No information avaliable
Vioxx H2O Solubility
Insoluble
Vioxx State
Solid
Vioxx LogP
3.019
Vioxx Dosage Forms
Tablet (12.5 mg, 25 mg, or 50 mg); Oral suspension (12.5 or 25 mg per 5 mL solution)
Vioxx Indication
For the treatment of osteoarthritis, acute pain in adults and menstrual pain.
Vioxx Pharmacology
Rofecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, is classified as a nonsteroidal anti-inflammatory drug (NSAID). Rofecoxib is used for its anti-inflammatory, analgesic, and antipyretic activities in the management of osteoarthritis (OA) and for the treatment of dysmenorrhea or acute pain. Unlike celecoxib, rofecoxib lacks a sulfonamide chain and does not require CYP450 enzymes for metabolism.
Vioxx Absorption
The mean oral bioavailability of rofecoxib at therapeutically recommended doses of 12.5, 25, and 50 mg is approximately 93%.
Vioxx side effects and Toxicity
No overdoses of rofecoxib were reported during clinical trials. Administration of single doses of rofecoxib 1000 mg to 6 healthy volunteers and multiple doses of 250 mg/day for 14 days to 75 healthy volunteers did not result in serious toxicity.
Vioxx Patient Information
PATIENT INFORMATION
Physicians should instruct their patients to read the patient package insert before starting therapy with VIOXX and to reread
it each time the prescription is renewed in case any information has changed.
VIOXX can cause discomfort and, rarely, more serious side effects, such as gastrointestinal bleeding, which may result in
hospitalization and even fatal outcomes. Although serious GI tract ulcerations and bleeding can occur without warning symptoms,
patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing
any indicative signs or symptoms. Patients should be apprised of the importance of this follow-up. Risk of GI Ulceration, Bleeding
and Perforation. Patients should be informed that VIOXX is not a substitute for aspirin for cardiovascular prophylaxis because of
its lack of effect on platelets. For additional cardiovascular safety information see CLINICAL STUDIES, Special Studies, VIGOR and
PRECAUTIONS, Cardiovascular Effects.
Patients should promptly report signs or symptoms of gastrointestinal ulceration or bleeding, skin rash, unexplained weight gain,
edema or chest pain to their physicians.
Physicians should instruct their patients to read the patient package insert before starting therapy with VIOXX and to reread
it each time the prescription is renewed in case any information has changed.
VIOXX can cause discomfort and, rarely, more serious side effects, such as gastrointestinal bleeding, which may result in
hospitalization and even fatal outcomes. Although serious GI tract ulcerations and bleeding can occur without warning symptoms,
patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing
any indicative signs or symptoms. Patients should be apprised of the importance of this follow-up. Risk of GI Ulceration, Bleeding
and Perforation. Patients should be informed that VIOXX is not a substitute for aspirin for cardiovascular prophylaxis because of
its lack of effect on platelets. For additional cardiovascular safety information see CLINICAL STUDIES, Special Studies, VIGOR and
PRECAUTIONS, Cardiovascular Effects.
Patients should promptly report signs or symptoms of gastrointestinal ulceration or bleeding, skin rash, unexplained weight gain,
edema or chest pain to their physicians.
Vioxx Organisms Affected
Humans and other mammals