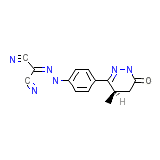
Trimazide




Trimazide Brand names, Trimazide Analogs
Trimazide Brand Names Mixture
- Alphasel tablets (calcium phosphate [dibasic] + cobalt sulfate + D-alpha tocopheryl acetate + selenium sulfide)
- Previte (neomycin sulfate + selenium sulfide + streptomycin sulfate + vitamin A + vitamin D + vitamin E + vitamin K1)
Trimazide Chemical_Formula
C14H12N6O
Trimazide RX_link
No information avaliable
Trimazide fda sheet
Trimazide msds (material safety sheet)
Trimazide Synthesis Reference
No information avaliable
Trimazide Molecular Weight
280.285 g/mol
Trimazide Melting Point
No information avaliable
Trimazide H2O Solubility
No information avaliable
Trimazide State
Solid
Trimazide LogP
2.759
Trimazide Dosage Forms
Intravenous (loading dose of 12 to 24 micrograms/kg over 10 minutes followed by a continuous infusion of 0.05 to 0.2 micrograms/kg per minute, adjusted according to response)
Trimazide Indication
For short term treatment of acutely decompensated severe chronic heart failure (CHF).
Trimazide Pharmacology
Levosimendan is a new Ca2+-sensitizing inotropic agent. Ca2+ sensitizers represent a new class of inotropic agents, which overcome the disadvantages associated with currently available inotropic agents in as they are not associated with an increased risk of arrhythmias, cell injury and death due to Ca2+ overload in myocardial cells; they do not increase the activation energy; and they have the potential to reverse contractile dysfunction under pathophysiologic conditions, such as acidosis or myocardial stunning. Levosimendan has not been approved for use in the U.S. or Canada.
Trimazide Absorption
The bioavailability of oral levosimendan is 85 ± 6% in healthy volunteers and 84 ± 4% in patients.
Trimazide side effects and Toxicity
No information avaliable
Trimazide Patient Information
No information avaliable
Trimazide Organisms Affected
Humans and other mammals