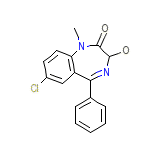
Restoril




Restoril Brand names, Restoril Analogs
Restoril Brand Names Mixture
- No information avaliable
Restoril Chemical_Formula
C16H13ClN2O2
Restoril RX_link
http://www.rxlist.com/cgi/generic/temaz.htm
Restoril fda sheet
Restoril msds (material safety sheet)
Restoril Synthesis Reference
E. Reeder et al., U.S. Pat. 3,340,253 (1967)
Restoril Molecular Weight
300.739 g/mol
Restoril Melting Point
119-121oC
Restoril H2O Solubility
164 mg/L
Restoril State
Solid
Restoril LogP
2.479
Restoril Dosage Forms
Capsule (7.5 mg, 15 mg, and 30 mg)
Restoril Indication
For the short-term treatment of insomnia (generally 7-10 days).
Restoril Pharmacology
Temazepam is a benzodiazepine used as a hypnotic agent in the management of insomnia. Temazepam produces CNS depression at limbic, thalamic, and hypothalamic levels of the CNS. Temazepam increases the affinity of the neurotransmitter gamma-aminobutyric acid (GABA) for GABA receptors by binding to benzodiazepine receptors. Results are sedation, hypnosis, skeletal muscle relaxation, anticonvulsant activity, and anxiolytic action.
Restoril Absorption
Well absorbed, minimal first-pass metabolism.
Restoril side effects and Toxicity
No information avaliable
Restoril Patient Information
Restoril Organisms Affected
Humans and other mammals